The Role of Patient Advocacy in the AdComm Process
From January 2024 to July 2024, the Federation of American Scientists interviewed 30 current and former Advisory Committee (AdComm) members. Based on these discussions, we were able to source potential policy recommendations that may assist with enhancing the FDA’s ability to obtain valuable advice for evidence-based decision-making. The results of these discussions are presented in case study format detailing the recurring themes that emerged and policy recommendations for improvement.
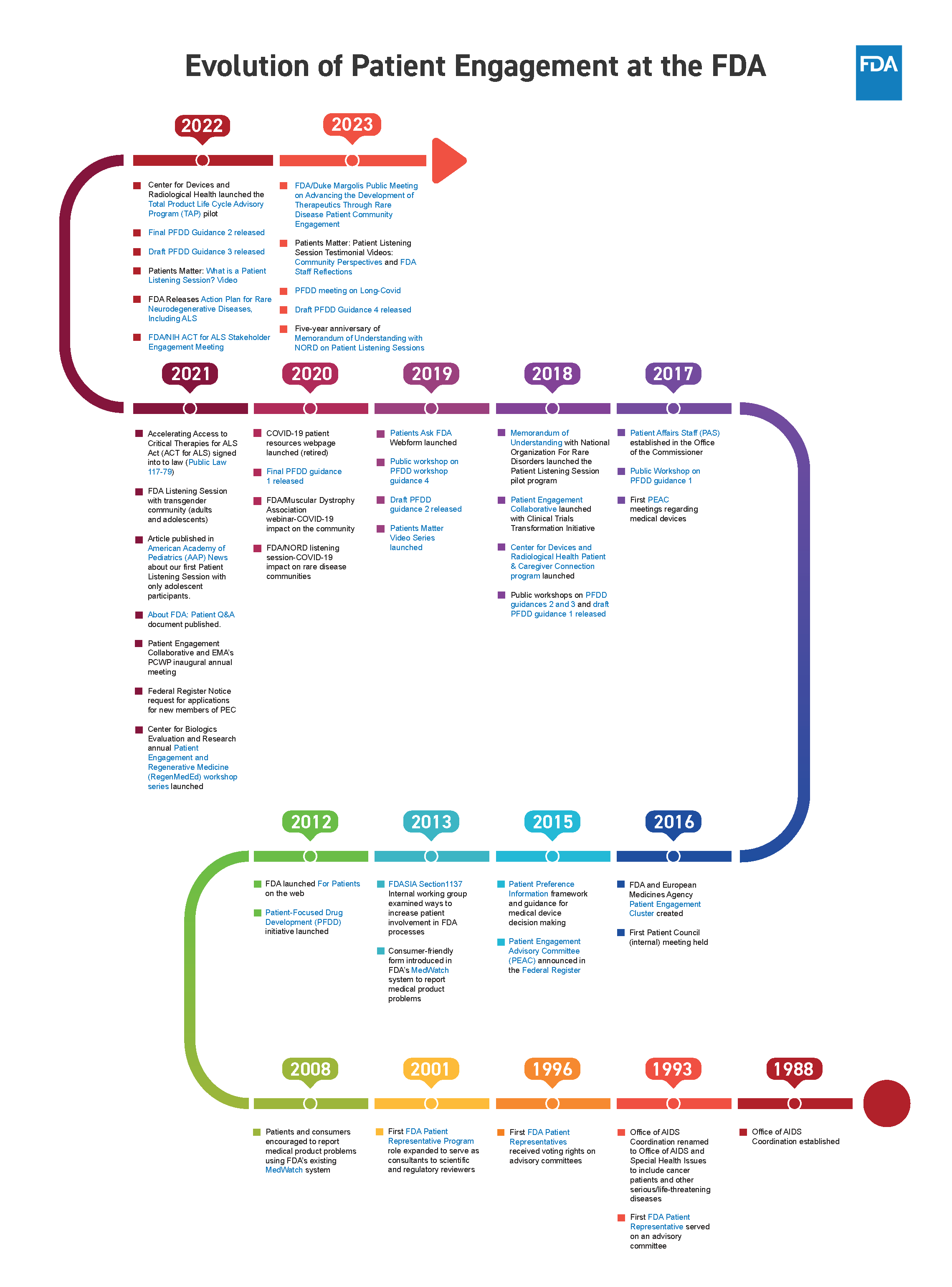
The regulation of medical products is the responsibility of the Food and Drug Administration (FDA). To ensure effective decision-making regarding these products, the FDA recognizes the importance of patient advocacy and the perspectives of patients. In 1988, the FDA initiated the patient engagement process through the Office of Aids Coordination, and within five years, the first patient representative was appointed to an FDA Advisory Committee. Since then, the FDA has significantly enhanced its methods of engaging patients, caregivers, and patient advocates. This includes the establishment of various offices, programs, collaboratives, listening sessions, public guidance, and more.
The FDA employs several avenues to engage patients in the regulatory process. Some avenues include the Patient Engagement Advisory Committee (PEAC), public comment, the Patient Focused Drug Development Initiative (PFDD), the Patient Listening Session Program, Patient Engagement Collaborative (PEC), and the Patient Representative Program (PRP). This list does not cover all the ways in which the FDA engages patients and advocates but provides an overview of the key operations involved in patient engagement efforts.
The Patient Engagement Advisory Committee is the only Committee that is completely composed of caregivers, patients, and patient representatives from various organizations, as a way to ensure that the lived experiences of these populations and their opinions are included in the deliberations and regulatory decision-making of medical products. Public comment is a requirement by law for federal agencies, allowing the public to provide feedback on proposed actions or new rules and regulations. Public comment is also sought during Advisory Committee meetings to gather information and perspectives from the public. PFDD meetings provide a platform for the FDA to obtain insights from patients on specific diseases and their treatments. To identify the issues most important to patients, the FDA has a series of guidance documents that are used specifically for PFDD meetings.
The Patient Listening Session Program facilitates informal meetings between patients, their representatives, and FDA staff. These sessions cover a range of topics, including treatment preferences, quality of life, unmet medical needs, and the impact of diseases and their symptoms. The PEC offers a forum for patients, caregivers, and advocates to discuss patient engagement operations. Lastly, the PRP allows patients, caregivers, and advocates who serve as special government employees the opportunity to provide advice to the FDA’s Commissioner or a designated representative on matters related to medical devices and their regulation.
Although there are various avenues for patient engagement and advocacy participation in the medical product regulation process, there are also ways in which these avenues can be expanded or improved.
Patient Advocacy Problems
For many years, patients and their caregivers have not seen significant or sustainable treatments that have been developed to treat many illnesses and diseases. Some treatments have proven to be ineffective yet still made it to market approval. On the other hand, there are treatments that met safety and efficacy standards but were not approved. There are also those treatments that are simply not affordable to the populations that need them most. In many of these scenarios, the patient representative voice was lost as they did not have the option to express their concerns or perspectives on certain treatments with decision makers. This further confirms that the role of patient advocacy and allowing space for the patient representative voice is crucial to the regulation process of medical products.
At the moment, the patient voice is not always heard because there are some FDA Advisory Committees that do not have a patient representative.
While the role of patient advocacy is crucial, it is important to note there should be boundaries in which patient perspective is considered for decision-making. Although patients and their advocates seek treatments that better address their needs, this desire can sometimes obscure their judgment concerning long-term treatment effectiveness. Frequently, patients and their supporters present powerful arguments to Advisory Committees and the FDA for approval of particular medical products which can lead to expedited medical product approval in the absence of supportive evidence.
The endorsement of eteplirsen, which was intended for the treatment of Duchenne muscular dystrophy (DMD), illustrates this point. Despite a 7 to 3 vote by the FDA’s Advisory Committee against approval due to insufficient evidence of its benefits, opposition from the FDA’s Center for Drug Evaluation and Research’s former leader resulted in the drug’s authorization. This sparked substantial internal and public criticism and led Dr. Ellis Unger from the FDA’s Office of Drug Evaluation to challenge the approval decision. Dr. Unger emphasized that “patient-focused drug development is about listening to patient perspectives about what matters to them; it is not about basing drug approvals on anecdotal testimony that is not corroborated by data.”
This approval was perceived by many as having been heavily influenced by patient advocacy and raised concerns about potential long-term implications for patient health. It also signaled a need to further examine both patient education and the appropriate limits of patient involvement in the regulatory process. This could have been mitigated had there been a list of criteria in place to be followed for public comment.
Incorporating Patient Perspectives
The Food and Drug Administration (FDA) is committed to understanding the balance of benefits and risks acceptable to patients as they relate to medical products. The FDA defines the role of patient representatives that serve on Advisory Committees as “Special Government Employees” who provide direct input to agency staff and share valuable insight on their experiences with various diseases, conditions, and devices while gaining access to confidential information. These representatives are selected by the FDA to serve on Advisory Committees using a specific set of criteria including, but not limited to:
- “Personal experience with the disease either as a patient or primary caregiver”
- “Knowledge about most treatment options and research for their areas of experience they are representing”
- “Impartiality and compliance with Federal ethics requirements (for example, financial interest, such as stock, in companies that may be affected by FDA decisions)”
This criteria ensures the FDA will understand the patient perspective as it relates to various medical products and ensures those selected to serve on Advisory Committees are knowledgeable about the areas in which they are aiming to provide guidance. Currently, there are some FDA Advisory Committees that do not have a patient representative. Further, the patient representatives serving on committees do not always have voting privileges. The absence of consistent voting privileges for some patient representatives on Advisory Committees and not having a standing patient representative on all committees hinders these individuals from providing an official stance on behalf of the community they represent. Additionally, public comment plays a significant role at Advisory Committee meetings by permitting individuals—including patients, caregivers, and advocacy organizations—to highlight concerns and propose solutions that may not have been previously considered by decision-makers. This process also helps the committee and agency gauge patient acceptance or opposition related to medical products, thereby enhancing their ability to make decisions that more accurately reflect public needs.
When Sarepta was seeking approval of eteplirsen for the treatment of DMD, a patient advocacy organization brought hundreds of patients, caregivers, and other advocates to the Advisory Committee convening so they could make a public comment to the Committee and the agency. Shortly after, the drug received a swift approval. Although it presented much controversy within the agency and the public, it showed how influential patient advocacy can be. Personal lived experiences, compelling stories of debilitating illnesses, and experiences with current treatment have the ability to impact regulatory decision-making.
The role of patient advocacy continues to be important in the Advisory Committee process and FDA regulatory decision-making process because it is crucial to assisting with decisions that affect the American public. Patient advocacy can be presented in the form of patient representatives that serve on Advisory Committees, those who make public comments during Advisory Committee convenings, and various outreach programs by advocacy organizations. The role of advocacy gives patients and caregivers support, promotes and protects their rights, and allows broader visibility for the issues that are most important to them. All of these avenues for patient perspective are important to understand how treatments perform, the current needs of the patient population, and how to tailor care for these populations by truly understanding their condition, diagnosis, and current management. Therefore, their voice is critical to truly understanding how various medical products will benefit their population, how they will access and afford these products, and how they will fill an unmet medical need.
Policy Recommendations
In an effort to better leverage Advisory Committee membership, the potential policy recommendations are as follows:
- Dedicate staff to identifying crucial public comments from patient advocates that should be considered for regulatory decision-making
- Include a patient representative on all committees that are reviewing medical products
- If possible, ensure that patient representatives selected have basic knowledge of the federal regulation process
- Establish criteria for which all public comments must abide by
- This can be done by creating a checklist for the public to review and consider before forming their comment and that clearly delineates the mandatory criteria that a medical product must meet to be considered for approval
- A disclaimer can also be added to state that there is a specific threshold or sample size of a population who must benefit from the medical product
- Promote patient focused medical product development through the use of incorporating patient perspectives into the life cycle of the regulatory process
- This can be done through hosting patient town hall discussions for areas such as rare diseases and expanding initiatives such as the Patient Focused Drug Development (PFDD) public meetings
- Make patient engagement ongoing, rather than only allowing patient engagement quarterly or annually as done with most FDA-led programs and initiatives
Conclusion
The landscape of disease burden and associated symptoms is ever-evolving. To ensure the FDA is best prepared for this changing landscape, patient advocacy and amplifying the patient voice should be considered vital to the development and regulation of medical products. Involving those who are the most impacted by these products is essential. The FDA can further promote the patient perspective and advance patient-centered health through incorporating patient representatives on all Committees that are reviewing medical products, making patient engagement an ongoing process, hosting town halls for patients to allow a broader audience the opportunity to voice opinions, and having dedicated staff to sort through public comments from patients.
Public meetings led by FDA Advisory Committees are instrumental in facilitating transparent deliberation between the FDA, the advisory body, and the American public.
Internal disagreements present a growing concern about FDA leadership overruling the expert opinions of scientific staff and proceeding with official approvals, thus undermining staff expertise, decreasing agency morale, and potentially diminishing public trust.
AdComm members note a lack of transparency in recruitment methods, insufficient training, and limited understanding of regulatory procedures.