Enhanced Household Air Conditioning Access Data for More Targeted Federal Support Against Extreme Heat
While access to cooling is the most protective factor against extreme heat events, the U.S. Census lacks granular, residential data to determine who has access to air conditioning (AC). The addition of a question about household access to working AC to the Census American Community Survey, a nationally representative survey on the social, economic, housing, and demographic characteristics of the population, would have life-saving impacts.
This is especially essential as the U.S. is experiencing more frequent and intense extreme heat events, and extreme heat now kills more people than all other weather-related hazards. Many vulnerable demographics — including people who are elderly, low-income, African-American, socially isolated, as well as those with preexisting health conditions— are exposed to high temperatures within their homes.
Better data on working AC infrastructure in American homes would improve how the federal government and its state and local partners target local social services and interventions, such as emergency responder deployment during high-heat events, as well as distribute federal assistance funds, such as the Weatherization Assistance Program (WAP), Low Income Home Energy Assistance Program (LIHEAP), and funding from the Inflation Reduction Act (IRA) along with the Bipartisan Infrastructure Law (BIL).
Challenge and Opportunity
In 2019, the U.S. Census Bureau acknowledged the danger of heat by issuing the Community Resilience Estimates (CRE) for Heat. The CRE for Heat is a measure that combines 10 questions from the existing American Community Survey questions. The questions ask about:
- Financial hardship
- Older residents living alone
- Crowding
- Whether the home is a mobile home, boat, or recreational vehicle
- Employment status for those under 65 years old
- Whether a resident has a disability
- Whether a resident has health insurance
- Access to a vehicle
- Connection via broadband internet access
- Communication barriers
However, the CRE for Heat lacks a question about air conditioning, the most important protective factor. Indoor temperature regulation is essential for mitigating heat illness and death on extremely hot days – temperatures above 86°F indoors can easily become dangerous and deadly.
Currently, the best information on residential AC is provided by the biennial American Housing Survey (AHS). In 2019, the AHS reported that 8.8% (11.6 million households) of all U.S. housing units have no form of AC. However, this information has three significant weaknesses. First, the American Housing Survey is based on 2,000 homes sampled across a metropolitan area. The sampling process generates an average across high-, medium-, and low-income residents; therefore, it overestimates the presence of AC in lower-income households. American households with higher incomes are more likely to have access to AC: 92.2% of households with incomes greater than $100,000 have some form of AC, compared with 88.9% of households with incomes less than $30,000. Second, lower-income households may have broken AC systems or units and lack money for repairs, skewing collected data. Third, the AHS fails to consider how poverty constrains electricity consumption. Many lower-income households reduce or abstain from using their AC in fear of costly electricity bills that trigger shutoffs. For instance, a 2022 report found that nearly 20% of households earning less than $25,000 reported keeping their indoor temperatures at levels that felt unsafe for several months of the year. These three weaknesses of the AHS data underscore the need for fine-grained information on who has access to working AC, especially in lower-income households.
The U.S. Census American Community Survey (ACS), on the other hand, samples 3.5 million addresses every year in a nationally representative annual survey. The ACS asks about housing characteristics, costs, and conditions (including heating) but not about AC nationwide. The equivalent survey administered in the four Island Areas of Guam, the Commonwealth of the Northern Mariana Islands, the U.S. Virgin Islands, and American Samoa — known as the “Island Areas Census” — included an AC question until 2010. This is an important precedent for adding a similar question to all Census surveys and should expedite the process. However, adding the term “working” (or a similar word) to the air-conditioning question would enhance its ability to capture low-income homes with broken systems as well as households that cannot use their existing AC due to energy insecurity.

Former question on air-conditioning in the American Community Survey for U.S. Island Areas
Better Information for Better Distribution of LIHEAP and WAP Funding
In addition to helping emergency responders, city planners, and public health departments, information collected on the presence of working AC could help ensure that the Department of Health and Human Services (HHS) Low Income Heat Energy Assistance Program (LIHEAP) and Department of Energy’s (DOE) Weatherization Assistance Program (WAP) serve the most vulnerable residents.
LIHEAP, administered by the Office of Community Services (OCS) within the Administration for Children and Families (ACF), is designed to “assist low-income households, particularly those in the lowest incomes, that pay a high proportion of household income for home energy, primarily in meeting their home energy needs.” LIHEAP is a targeted block grant program whereby states distribute their funds across three programs that subsidize home energy heating or cooling costs; fund payment in crises; and support home weatherization (limited to 15% of funds unless a state requests a waiver to increase their percentage to 25%). The largest proportion of the funds subsidizes lower-income, vulnerable residents’ energy spending. While LIHEAP is an important federal program that impacted 7.1 million American households in 2023, only approximately 20% of eligible households received LIHEAP assistance, and the program is currently facing budget shortfalls of $2 billion.
By expanding cooling assistance, LIHEAP is being asked to do more with less: 24 of 50 states now include cooling assistance, and 9.8% of funds subsidized cooling costs. As extreme heat events become more frequent and severe and households become more energy insecure in the face of rising energy prices, more states will need to expand cooling assistance programs. Data on where households are most vulnerable — that is, those households without working AC or the financial ability to operate their AC — would enable targeted distribution of federal funds. Therefore, adding a Census question on household access to working AC would provide critical information to ensure LIHEAP funds serve the most vulnerable households.
Unlike LIHEAP, WAP’s sole focus is weatherization. Many weatherization improvements that help in cold weather also improve indoor thermal comfort during warm summer months. These improvements include fixing broken AC; adding insulation in walls, attics, and crawlspaces; and replacing leaky, inoperable windows. Compared to LIHEAP, WAP serves a much smaller number of homes — 35,000 homes annually versus LIHEAP’s 7.1 million (as of FY2023). Knowing the number of individual households in a census tract in need of investments in heat resilience adaptation and air-conditioning would enable much more targeted delivery of limited federal resources. Further, DOE can use this information to predict future grid demand and enhance necessary resilience measures for hotter summers.
Plan of Action
To save lives in the face of growing extreme heat, the Census should add a question about working AC to the American Community Survey. This could be executed as follows:
Recommendation 1. The Office of Community Services in the Administration for Children and Families (OCS ACF) requests the addition of a question about access to working AC at the census tract level to the American Community Survey. This would directly aid the LIHEAP program’s mandate to identify and serve vulnerable individuals, and benefit other programs like DOE’s WAP as well as programs authorized by the IRA and BIL.
Recommendation 2. Legal staff in the Office of Management and Budget (OMB) and the Census Bureau review the proposal to determine whether it meets legislative requirements.
Recommendation 3. After a successful legal review, OMB and the Census Bureau, in consultation with the Interagency Council on Statistical Policy Subcommittee for the American Community Survey, determine whether the request merits consideration.
Recommendation 4. Subject matter experts across relevant federal government programs (i.e. LIHEAP and WAP) and external institutions (housing experts, extreme heat experts, social vulnerability experts) identify ways to ask the question. The Census Bureau conducts interviews to determine which wording produces the most accurate results. Because a similar question (but lacking the term “working”) is used on the American Community Survey for Island Areas, this process may be expedited. A potential example of the new question is below:
Do you have working air air-conditioning?
- Yes, a central air conditioning system (includes split-type)
- Yes, 1 individual room unit
- Yes, 2 or more individual room units
- No, my air conditioning system is non-functional or broken
- No, I cannot afford to run my air-conditioning system
- No, I do not have any air-conditioning system
Recommendation 5. The Census Bureau solicits public comment on the question and request OMB’s approval for field testing.
Recommendation 6. The Census Bureau and ACF OCS review the results and decide whether to recommend adding the new survey question. Through the Federal Register Notice, the Census Bureau solicits public comment. Public comments inform the final decision that is made in consultation with the OMB and the Interagency Council on Statistical Policy Subcommittee on the American Community Survey.
Recommendation 7. If approved by OMB, the Census Bureau adds the question to its materials, and implementation begins at the start of the following calendar year (October).
Recommendation 8. The Community Resilience Estimates (CRE) for Heat is updated with information about AC as it becomes available. This tool can be shared, along with refined guidance, with state-level administrators of programs like LIHEAP and WAP to target investments to the households most vulnerable to overheating and resulting heat illness and death. The CDC could integrate AC coverage within its existing syndromic surveillance programs on extreme heat, as an additional layer of “risk” for targeted public health deployment during high-heat events.
Conclusion
The U.S. lacks fine-scaled data to determine whether households can access working AC systems/units and operate them during extreme heat events. Adding a question to the American Community Survey will provide life-saving information for emergency responders, social service providers, and city staff as extreme heat events become more frequent and intense. This fine-scaled information will also aid in distributing LIHEAP and WAP funding and increase the federal government’s ability to protect the most vulnerable residents from life-threatening extreme heat events.
This idea of merit originated from our Extreme Heat Ideas Challenge. Scientific and technical experts across disciplines worked with FAS to develop potential solutions in various realms: infrastructure and the built environment, workforce safety and development, public health, food security and resilience, emergency planning and response, and data indices. Review ideas to combat extreme heat here.
Revolutionizing Research and Treatments for Infection-Associated Chronic Diseases
The National Institutes of Health should create an Office of Infection-Associated Chronic Illness Research. Reporting directly to the NIH Director, the Office would provide a timely and urgently needed command center for prioritizing innovative research across a group of complex, chronic conditions that are all known to be downstream effects of viral and bacterial infections. These include Long Covid and many others. The Office of IACIR should champion transformative, catalytic research that cuts across multiple institutes and centers.
The Covid-19 pandemic has proven to be a massive disabling event that has shined a bright and historic light on infection-associated illnesses. As many as 1 in 5 adults develops a new health condition in the aftermath of Covid, and for many, the condition could be lifelong. This should not come as a surprise. For decades, millions of sufferers have experienced debilitating illness, gaslighting, misunderstanding, lack of insurance coverage, disability, and no FDA-approved treatment options. In alignment with President Biden’s National Research Action Plan for Long Covid, the Office should pursue a two-pronged approach that includes pioneering next-generation diagnostics while also fast-tracking patient-centered clinical trials for repurposed drugs. The Office would spur creation of a world in which all people with an infection-associated chronic illness have access to a timely diagnosis and effective treatments.
Challenge and Opportunity
The world faces a massive problem with long term disability due to the long-term effects of infections. The cost of Long Covid is estimated at $3.7 trillion over five years, according to Harvard University economist David Cutler. Within the United States, it is estimated that up to 23 million Americans currently have or have had Long Covid or similar complex, chronic conditions.
Long Covid is part of a family of infection-associated chronic illnesses. More than two-thirds of people with Long Covid develop moderate to severe dysautonomia, most commonly presenting as postural orthostatic tachycardia syndrome (POTS), a condition estimated to impact up to 3 million Americans prior to the pandemic. Dysautonomia symptoms, the result of a problem with the autonomic nervous system, include lightheadedness, palpitations, profound fatigue, exercise intolerance, cognitive impairment, gastrointestinal dysmotility and more. Similarly, about half of all Long Covid cases fit the criteria for myalgic encephalomyelitis, or chronic fatigue syndrome (ME/CFS). With two of the most common symptoms of ME/CFS being unrelenting exhaustion and brain fog. These symptoms are also seen in persistent Lyme disease. Patients with Long COVID, dysautonomia/POTS, ME/CFS or persistent Lyme disease often present with autoimmunity, small fiber neuropathy, gut dysbiosis, migraine, mast-cell activation syndrome (MCAS), Ehlers Danlos syndrome (EDS), and cranio-cervical instability (CCI).
While there appears to be significant shared pathophysiology and symptomatology between these diseases, progress in each of these diseases has been stymied because research has been siloed and underfunded. For instance, one analysis of NIH funding and disease burden showed that ME/CFS received just 7% of research dollars commensurate with disease burden, making it the most underfunded disease at NIH with known disability-adjusted life years data. Reaching parity with diseases of similar severity and prevalence would require a fourteen fold increase in ME/CFS.
Each condition is in its own silo for a different reason, making full coordination impossible until a new NIH office is established. For instance, Gulf War illness doesn’t have an NIH budget line item at all; it is studied through the Department of Defense’s medical research program. And while the NIH studies acute Lyme infections, the agency didn’t formally start studying “post-treatment Lyme disease syndrome” until 2023. For POTS, there is a lack of research showing quality of life disruptions for dysautonomia sufferers. This makes it impossible to quantify the gap in research funding given the disorder’s large economic burden. And for decades, ME/CFS research was hamstrung in part because it was housed in NIH’s poorly funded Office of Research on Women’s Health. In short, to adapt a line from Leo Tolstoy’s Anna Karenina, “Understood diseases are all alike; every misunderstood disease is misunderstood in its own way.”
Therefore, studying infection-related conditions all together, within one multidisciplinary NIH office, provides an unprecedented scientific opportunity to build on existing research and apply a comprehensive molecular biology approach toward unraveling how the body’s systems go awry in complex disorders. Given the urgent need to rapidly scale interventions, these diseases also provide an ideal opportunity to make immediate progress with clinical trials for repurposed drugs.
This synergistic approach is also the most efficient and cost-effective from a financial standpoint, because it creates economies of scale and reduces redundancies that result from researching each disease piecemeal, from their respective silos. Streamlining research under one roof would also eliminate red tape and bureaucratic inefficiencies, thus ensuring the type of low barriers to entry and high return on investment (ROI) that are necessary to attract private sector participation. Moreover, a plan to fast-track FDA approval of promising drug therapies would both incentivize pharmaceutical involvement and guarantee that patients receive life-changing treatments as quickly as possible.
ME/CFS is an often lifelong condition in which about half of all patients are disabled and cannot work full-time. The level of disability for ME/CFS has been compared to that of cancer, heart disease, and last-stage AIDS. POTS is also often a lifelong condition, with a majority of patients reporting symptoms staying the same or worsening over time. Health-related quality-of-life in POTS is worse than what is seen in diabetes, neoplasms, cardiovascular disease, COPD, HIV and chronic kidney disease. Less than half of adult POTS patients are employed, and of those who are able to work, POTS symptoms prevent a majority of them from working as many hours as they would like to work. More than half of college students with POTS drop out of college due to the severity of their POTS symptoms. Given the high rate of POTS and ME/CFS with the Long Covid population, it follows that Long Covid patients can expect a similar prognosis. For all three diagnoses, there are as yet no treatments approved by the Food and Drug Administration. The landscape for drugs to treat these conditions is also undeveloped.
Given the magnitude of the challenge, a realistic budget for a Long Covid “moonshot” should be at least $1 billion per year for 10 years. Therefore, to incorporate all infection-associated chronic illnesses, the budget would need to be a great deal higher. This is an historic opportunity for the U.S. to lead with state-of-the art scientific research. A fully funded and comprehensive research program can tackle these diseases, alleviate suffering, and enable these individuals once again to pursue their dreams as productive members of society.
Several NIH offices created in recent years show us how to seize the current opportunity. In response to the most recent previous global pandemic, HIV/AIDS, the NIH created the Office of AIDS Research in 1988.
Later, the NIH established the Office of Women’s Health Research in 1990, after the Congressional Caucus for Women’s Issues asked the General Accounting Office to conduct an investigation into NIH’s implementation of guidelines for inclusion of women in medical research. The OWHR remedies longstanding inequities in which women were dramatically underrepresented in clinical research.
More recently, in 2023, the NIH launched its Office of Autoimmune Research. The office was originally proposed by then-Senator Joe Biden in 1999. In 2022, the National Academy of Sciences, Engineering, and Medicine held a research symposium, and issued a conclusive report, outlining five options for how to elevate federal research on autoimmune disease.
One of those called for the establishment of the Office, situated within the Office of the Director. The authors noted the benefits of that high-level placement, including elevated visibility, sustained leadership, and becoming a clear focal point for intramural, extramural, training, and outreach activities. Placing it close to the NIH Director “may provide many of the benefits of a new Institute…with fewer bureaucratic costs or controversies,”they wrote.
On June 29-30, 2023, NASEM held a similar symposium to begin establishing a common research agenda for infection-associated chronic illnesses. The creation of the new Office of IACIR should organically flow out of this past summer’s NASEM meeting, just as the Office of Autoimmune Research did from the 2022 meeting.
Last year’s NASEM symposium was a watershed moment in the history of chronic illness patient advocacy movements, which for decades had effectively been voices in the wilderness. The nation’s most esteemed scientific body had consolidated the foundational literature for each condition, identified the possibilities for common pathophysiology, and illuminated a path forward. This establishes a clear generational opportunity to solve a major set of disabling conditions globally, and positions American institutions to lead in pioneering these breakthroughs.
Plan of Action
Working with champions in Congress, a select group of Administration officials – across Office of Science and Technology Policy, Domestic Policy Council, NIH, and the HHS Assistant Secretary for Health – would serve as executive sponsors and provide oversight.
Each of these primary stakeholders should take responsibility for the following steps in executing this proposal.
Clearly state the goals of the office and its NIH-wide responsibilities.
Since this research must span neurology, immunology, cardiology, pulmonology, virology, and other fields to encompass the multi-system impact of these illnesses, the Office must have a clearly-defined mission and authority to integrate work across multiple NIH institutes.
The key functions of the Office should include:
- Evangelize the concept of infection-associated chronic illnesses to the public, health providers, and researchers and administrators inside NIH
- Ensure that NIH and health systems are responsive to long-term sequelae of current and future pandemics
- Serve as a convener for industry, disease organizations, patient advocates, and patients across IACIs to set research priorities and design studies
- Embrace a spirit of co-producing research with patients, acknowledging the wisdom that those with lived experience bring to the scientific enterprise,
- Advance state-of-the-art IACI research focusing on biomarkers, root causes, and therapeutic targets in collaboration with patient communities
- Hold budgetary authority to fund and coordinate IACI research across all Institutes
- Identify and validate common biomarkers and therapeutic targets across conditions
- Collaborate with other U.S. government agencies (FDA, CDC, SSA, AHRQ, PCORI, etc.), community groups, and global organizations to catalyze rapid diagnosis, effective treatment, and relevant disability services/supports for all IACI patients
- Advise Director of NIH, HHS, and other entities on IACI research. In particular, this Office should directly coordinate with HHS’s Office of Long Covid Research and Practice so that IACI research is synergistic with a cabinet-level champion
Identify leadership and staffing.
At minimum, the office would require robust staffing and could be funded through several avenues.
To begin, the Office of IACIR’s authority could be inaugurated under the auspices of the NIH’s Common Fund. This is a highly attractive option because it wouldn’t require additional Congressional funding allocations. The fund creates a space where investigators across NIH institutes collaborate on innovative research in order to address high-priority challenges and make a broader impact on the scientific community. Among the Common Fund’s most successful initiatives is the Undiagnosed Diseases Network.
To best amplify its mission, the office should be placed within the Office of the Director. Importantly, we stipulate that the NIH Director leads this new Office in consultation with community stakeholders, who have decades of experience managing infection-associated chronic conditions.
Congress could also consider bicameral legislation to create this new NIH office. If passed, policymakers could consider taking approaches similar to those taken for AIDS and Alzheimer’s, which could mandate special oversight of this Office. AIDS legislation, for instance, requires NIH to submit a research plan directly to Congress. Alternatively, Congress should also use the authority of the Congressionally Directed Medical Research Program to support and oversee this Office.
Launch a comprehensive IACI research agenda.
The Office should create a high-level blueprint as well as a more detailed agenda with an implementation plan for carrying it out. Research projects should mirror the most recent findings and avenues for next steps discussed at the NASEM symposium.
Diagnostic research activities should include:
- Advanced central and peripheral nervous system analysis and imaging
- AI-based analysis of immune profiles and comprehensive panels
- Investigation of viral or bacterial persistence, microclotting/coagulation parameters, tissue pathology, and epigenetics
Clinical trial platforms should support state-of-the-art techniques including:
- Decentralized, multi-arm clinical trials with dynamic, adaptable design
- Cross-diagnosis research amongst IACIs with common co-morbidity
- More efficient testing of repurposed medications
Not only would these approaches incorporate best practices scientifically, but by combining multiple diseases into single studies, they would create economic efficiencies that would reduce costs overall and make it easier and more cost-effective to roll out treatments.
Scale it into an Institute.
Once the new Office becomes established in the NIH and has put “points on the board” with early successes in its first five years, leaders at NIH and in the Administration should evaluate how to develop it into a Center or Institute. Alternatively, Congress could pass further legislation to elevate it to the level of an Institute.
An Institute is likely the best vehicle to fully execute a true long-term high investment capable of curing these diseases. Given the economic and social burden of these diseases – and coupled with their historic neglect – an annual research budget measured in the billions of dollars may be required.
Conclusion
Throughout its history, the NIH has continually evolved to meet new and pressing challenges as scientific understanding has progressed. Globalization, microbial resistance, and climate change continue to upset the balance of the natural world, with unpredictable effects on the human population. It’s not a question of if – but rather when – the next global pandemic will occur. Every pandemic causes long-term health consequences. The research advanced by this Office would foster pandemic resilience against the types of global infectious threats that will become increasingly common in the modern world. At the same time, it would help address the large swath of disability from the trickle-down of chronic illnesses triggered by everyday community infections as well.
Just as the NIH Office of AIDS Research has made great strides against AIDS, a new Office of Infection-Associated Chronic Illness Research will turn the tide against Long Covid and its many cousins. By diagnosing, managing, treating and ultimately curing these conditions, this program will help many millions get their lives and careers back. As they rejoin the workforce and contribute to the economy, the returns generated by this Office will exceed its costs by many orders of magnitude.
The Office of IACIR should dynamically collaborate with several offices at the cutting edge. First among these is the Office of Long Covid Research and Practice, established in 2023 under the Office of the Assistant Secretary for Health (OASH), includes an advisory committee composed of as many as 20 members.
Next, our future NIH Office should work in partnership with the federal government’s new health moonshot agency – the Advanced Research Projects Agency for Health (ARPA-H) – which is uniquely suited to help lead on building next-generation diagnostics for infection-associated chronic illnesses. Its model calls for rapid high-risk, high-reward science. Launched in 2022, ARPA-H is currently hiring its first slate of program managers, leading innovative projects that are disease-agnostic. Infection-associated chronic illnesses could be a target of a future ARPA-H program manager.
The Office should work closely with the Food and Drug Administration, such that safe and effective repurposed drugs can be approved for this patient population.
And throughout all of this, the Office must collaborate with the Patient Centered Outcomes Research Institute (PCORI), which has funded innovative work by the Patient Led Research Collaborative on Covid-19 to develop patient scorecards to grade the efficacy and quality of research proposals. To improve equity and stakeholder engagement, NIH should consider piggybacking off such efforts.
- Establish a consensus vocabulary; assess which chronic diseases or illnesses are “infection-associated,” and potentially expand into more areas
- Annually develop and evaluate a strategic plan for all IACI research across NIH Institutes, Centers, and Offices
- By the end of its first year, hold an international conference to rapidly develop a common research agenda, timeline, and milestones toward key accomplishments by 2030
- Accelerate development of a common IACI biobank by leveraging existing disease-specific biobanking initiatives
- Build research infrastructure to seed and sustain diverse and multidisciplinary IACI scientific workforce
- Establish advisory council for whole-of-government approach to IACI research, care, and services
- Involve and incentivize the private sector and fast-tracking FDA approval for promising drugs
How an Obscure Law Shapes the Way the Public Engages with the Food and Drug Administration
Every day, the executive branch of the federal government makes transformative policy changes. When federal agencies need expert input, they look to advice from external experts and interested citizens through a series of public engagement mechanisms, from public meetings to public comment. Of these, only one mechanism allows the executive branch to actively source consensus-based public advice and for external experts to directly advise policymakers, the Federal Advisory Committee Act (FACA). And it’s a law many Americans have never heard of.
FACA enables agencies to create advisory committees
Enacted in 1972, FACA governs expert and public engagement with executive branch decision making. FACA articulates rules for the establishment, operation, and termination of advisory committees (AC), groups of experts that the federal agencies establish, manage, and use to provide external advice on key policy questions. At any given moment in time, there are ~1000 active ACs across the federal government making crucial recommendations to agency leaders.
At the Food and Drug Administration (FDA), FACA is essential to the workings of the agency’s regulatory engine and public health mission. The FDA uses its ACs to provide independent advice on medical products (drugs and devices), providing a unique window for experts and the public to comment on cutting-edge medical products in the approvals pipeline. ACs capture the headlines through their “yes” or “no” votes on product approval, raising spirits or breaking hearts. Industry takes notice: medical product sponsors spend months preparing for these meetings, supported by a boutique industry geared to help them “ace” their AC meetings.
ACs need to be reformed to build public trust in the FDA
While ACs are a crucial transparency measure for an agency like FDA that is currently grappling with declining public trust, the system has been repeatedly under fire. Recent controversies include FDA’s public overruling of AC recommendations against approval for hydrocodone, an opioid pain reliever, and aducanumab, an Alzheimer’s treatment. After aducanumab approval, several high-profile resignations exacerbated the trust-issues. What’s more, FDA’s use of ACs is in decline, with the percentage of new drugs reviewed by ACs decreasing by almost 10 times from 2010-2021. These actions are in direct conflict with current whole-of-government efforts to modernize regulatory review and expand meaningful participation in the regulatory decision making process. Advancing racial equity, opening up the scientific enterprise, and broadening public engagement in regulatory decisions will require transformative policy solutions for the FDA.
To re-envision how the FDA and other federal agencies engage external scientific experts and the public to address critical challenges facing public health, FAS is diving deep into how FACA is put into action at the FDA. Over the next year, FAS will be engaging AC members on their experiences in service, understanding key evidence needs at the agency that a reformed AC system could better meet, and scoping necessary process, regulatory, and statutory changes to the AC system. This will build upon our previous efforts: FAS has participated in and provided public comment to many AC meetings and documented how ACs are slow to respond to emerging questions of regulatory concern in our ongoing work to address bias in medical innovation. FAS has also documented strategies to improve science advice for the executive branch, including FACA reform. We invite you to follow this work and join us in calling for reforms that strengthen trust in the FDA Advisory Committee system.
Calls for systematic reform are coming from leadership across the FDA, yet consensus does not yet exist on what those reforms should look like. From recommendations to get rid of voting requirements at meetings (already receiving Congressional scrutiny), to broadening membership, including to members with conflicts of interest, to increasing review timelines of sponsor materials before meetings, there is no shortage of ideas for what this new system could look like. Non-profit leaders and academic researchers have also started coming together to make recommendations that address FDA’s influence over Advisory Committee discussions and ongoing issues with agency leadership overruling the AC’s vote. There could also be clearer requirements for the FDA to respond to AC recommendations and make set public timelines for agency action. Twenty-five Attorneys General recently called on the FDA to release updates to its actions on pulse oximetry one year after the AC meeting.
More broadly, the FDA can learn from other agencies with explicit policies guiding their public engagement, such as the Meaningful Involvement Policy at the Environmental Protection Agency. These FDA-specific recommendations build upon long-standing calls to reform FACA to reduce the administrative barriers that make it challenging to solicit expert advice when needed or lead some agencies to forgo processes that could invoke FACA altogether.
To improve patient care, it is essential to create a nimble, participatory, and transparent process that ensures regulated products will benefit the health of all Americans. AC reform will be essential to building the FDA’s capacity to address increasingly complex regulatory science challenges, from artificial intelligence, to real-world data, to emerging platform technologies, to health inequity, while also improving the federal government’s ability to more rapidly generate consensus-based science advice. FAS is excited to play our part in strengthening evidence-based policy by engaging in policy entrepreneurship to engage stakeholders, develop roadmaps, and advocate for change.
Moving the Nation: The Role of Federal Policy in Promoting Physical Activity
Physical activity is one of the most powerful tools for promoting health and wellbeing. Movement is not only medicine—effective at treating a range of physical and mental health conditions—but it is also preventive medicine, because movement reduces the risk for many conditions ranging from cancer and heart disease to depression and Alzheimer’s disease. But rates of physical inactivity and sedentary behavior have remained high in the U.S. and worldwide for decades.
Engagement in physical activity is impacted by myriad factors that can be viewed from a social ecological perspective. This model views health and health behavior within the context of a complex interplay between different levels of influence, including individual, interpersonal, institutional, community, and policy levels. When it comes to healthy behavior such as physical activity, sustainable change is considered most likely when these levels of influence are aligned to support change. Every level of influence on physical activity within a social-ecological framework is directly or indirectly affected by federal policy, suggesting physical activity policy has the potential to bring about substantial changes in the physical activity habits of Americans.
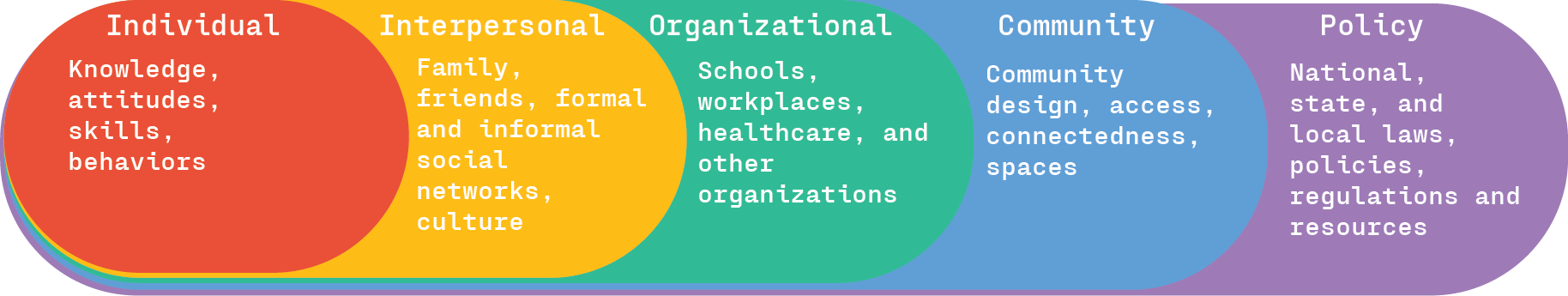
Why are federal physical activity policies needed?
Physical inactivity is recognized as a public health issue, having widespread impacts on health, longevity, and even the economy. Similar to other public health issues over past decades such as sanitation and tobacco use, federal policies may be the best way to coordinate large-scale changes involving cooperation between diverse sectors, including health care, transportation, environment, education, workplace, and urban planning. An active society requires the infrastructure, environment, and resources that promote physical activity. Federal policies can meet those needs by improving access, providing funding, establishing regulations, and developing programs to empower all Americans to move more. Policies also play an important role in removing barriers to physical activity, such as financial constraints and lack of safe spaces to move, that contribute to health disparities. With such a variety of factors impacting active lifestyles, physical activity policies must have inter-agency involvement to be effective.
What physical activity initiatives exist currently?
Analysis of publicly available information revealed that there are a variety of initiatives currently in place at the federal level, across several departments and agencies, aimed at increasing physical activity levels in the U.S. Information about each initiative was evaluated for their correspondence with levels of the social-ecological model, as summarized in the table. Note that it is possible the search that was conducted did not identify every relevant effort, thus there could be additional initiatives that are not included below.
Given the large number of groups with the shared goal of increasing physical activity in the nation, a memorandum of understanding (MOU) may help to promote coordination of goals and implementation strategies.
These and other federal departments and agencies can coordinate action with state and local partners, for example in healthcare, business and industry, education, mass media, and faith-based settings, to implement physical activity policies.
The CDC’s Active People, Healthy Nation initiative provides an example of this approach. This campaign, launched in 2020, has the goal of helping 27 million Americans become more physically active by 2027. By taking action steps focused on program delivery, partnership engagement, communication, training, and continuous monitoring and evaluation, the campaign seeks to help communities implement evidence-based strategies across sectors and settings to provide equitable and inclusive access to safe spaces for physical activity. According to our analysis, the strategies of the Active People, Healthy Nation initiative are aligned with the social-ecological model. The Physical Activity Policy Research and Evaluation Network, a research partner of the Active People, Healthy Nation initiative, provides an example of coordinating with partners in other sectors to promote physical activity. Through collaboration across sectors, the network brings together diverse partners to put into practice research on environments that maximize physical activity. The network includes work groups focused on equity and inclusion, parks and green space, rural active living, school wellness, transportation policy and planning, and business/industry.
The Biden-Harris Administration National Strategy on Hunger, Nutrition, and Health, announced in September 2022, also includes strategies that are consistent with a social-ecological model. The strategy outlines steps toward the goal of ending hunger and increasing healthy eating and physical activity by 2030 so that fewer Americans will experience diet-related diseases. Pillar 4 of the strategy is to “make it easier for people to be more physically active—in part by ensuring that everyone has access to safe places to be active—increase awareness of the benefits of physical activity, and conduct research on and measure physical activity.” The strategy specifies goals such as building environments that promote physical activity (e.g., connecting people to parks; promoting active transportation and land use policies to support physical activity) and includes a call to action for a whole-of-society response involving the private sector, state, local, and territory governments, schools, and workplaces.
The Congressional Physical Activity Caucus has been active in introducing legislation that can help realize the goals of the current physical activity initiatives. For example, in February 2023, Sen. Sherrod Brown (D-OH), co-chair of the Caucus, introduced the Promoting Physical Activity for Americans Act, a bill that would require the Department of Health and Human Services to continue issuing evidence-based physical-activity guidelines and detailed reports at least every 10 years, including recommendations for population subgroups (e.g., children or individuals with disabilities). In addition, members of the Caucus, along with other members of congress, reintroduced the bipartisan, bicameral Personal Health Investment Today (PHIT) Act in March 2023. This legislation seeks to encourage physical activity by allowing Americans to use a portion of the money saved in their pre-tax health savings account (HSA) and flexible spending account (FSA) toward qualified sports and fitness purchases, such as gym memberships, fitness equipment, physical exercise or activity programs and youth sports league fees. The bill would also allow a medical care tax deduction for up to $1,000 ($2,000 for a joint return or a head of household) of qualified sports and fitness expenses per year.
What progress has been made?
There are signs that some of the national campaigns are leading to changes at other levels of society. For example, 46 cities, towns, and states have passed an Active People, Healthy Nation Proclamation as of September 2023. According to the State Routes Partnership, which develops “report cards” for states based on their policies supporting walking, bicycling, and active kids and communities, many states have shown movement in their policies between 2020 and 2022, such as implementing new policies to support walking and biking and increasing state funding for active transportation. However, more time is needed to determine the extent to which recent initiatives are helping to create a more active country, since most were initiated in the past two or three years. Predating the current initiatives, the overall physical activity level of Americans increased from 2008 to 2018, but there has been little change since that time, and only about one-quarter of adults meet the physical activity guidelines established by the CDC.
Clearly, there is a critical need for concerted effort to implement the strategies outlined in current physical activity initiatives so that national policies have the intended impacts on communities and on individuals. Leveraging provisions in existing legislation related to the social-ecological model of physical activity promotion will also help with implementation. For example, title III-D of the Older Americans Act supports healthy lifestyles and promotes healthy behaviors amongst older adults (age 60 and older), providing funding for evidence-based programs that have been proven to improve health and well-being and reduce disease and injury. Physical activity programs are prime candidates for such funding. In addition, programs under the 2021 Bipartisan Infrastructure Law and the 2022 Inflation Reduction Act are helping to change the current car-dependent transportation network, providing healthier and more sustainable transportation options, including walking, biking, and using public transportation, and are providing investments in environmental programs to improve public health and reduce pollution. For example, states can use funds from the Highway Safety Improvement Program for bicycle and pedestrian highway safety improvement projects, and funding is available through the Carbon Reduction Program for programs that help reduce dependence on single-occupancy vehicles, such as public transportation projects and the construction, planning, and design of facilities for pedestrians, bicyclists, and other non-motorized forms of transportation.
Partnering with non-governmental groups working towards common goals, such as the Physical Activity Alliance, can also help with implementation. The Alliance’s National Physical Activity Plan is based on the socio-ecological model and includes recommendations for evidence-based actions for 10 societal sectors at the national, state, local and institutional levels, with a focus on making change at the community level. The plan shares many priorities with those of the Active People, Healthy Nation initiative, while also introducing new goals, such as establishing a CDC Office of Physical Activity and Health.
With coordinated action based on established public health models, such as the social-ecological framework, federal policies can be successfully implemented to make the systemic changes that are needed to create a more active nation.
The work for this blog was undertaken before Dr. Dotson joined the Agency for Healthcare Research and Quality (AHRQ). Dr. Dotson is solely responsible for this blog post’s contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Readers should not interpret any statement as an official position of AHRQ or of the U.S. Department of Health and Human Services.
It’s Time to Move Towards Movement as Medicine
For over 10 years, physical inactivity has been recognized as a global pandemic with widespread health, economic, and social impacts. Despite the wealth of research support for movement as medicine, financial and environmental barriers limit the implementation of physical activity intervention and prevention efforts. The need to translate research findings into policies that promote physical activity has never been higher, as the aging population in the U.S. and worldwide is expected to increase the prevalence of chronic medical conditions, many of which can be prevented or treated with physical activity. Action at the federal and local level is needed to promote health across the lifespan through movement.
Research Clearly Shows the Benefits of Movement for Health
Movement is one of the most important keys to health. Exercise benefits heart health and physical functioning, such as muscle strength, flexibility, and balance. But many people are unaware that physical activity is closely tied to the health conditions they fear most. Of the top five health conditions that people reported being afraid of in a recent survey conducted by the Centers for Disease Control and Prevention (CDC), the risk for four—cancer, Alzheimer’s disease, heart disease, and stroke—is increased by physical inactivity. It’s not only physical health that is impacted by movement, but also mental health and other aspects of brain health. Research shows exercise is effective in treating and preventing mental health conditions such as depression and anxiety, rates of which have skyrocketed in recent years, now impacting nearly one-third of adults in the U.S. Physical fitness also directly impacts the brain itself, for example, by boosting its ability to regenerate after injury and improving memory and cognitive functioning. The scientific evidence is clear: Movement, whether through structured exercise or general physical activity in everyday life, has a major impact on the health of individuals and as a result, on the health of societies.
Movement Is Not Just about Weight, It’s about Overall Lifelong Health
There is increasing recognition that movement is important for more than weight loss, which was the primary focus in the past. Overall health and stress relief are often cited as motivations for exercise, in addition to weight loss and physical appearance. This shift in perspective reflects the growing scientific evidence that physical activity is essential for overall physical and mental health. Research also shows that physical activity is not only an important component of physical and mental health treatment, but it can also help prevent disease, injury, and disability and lower the risk for premature death. The focus on prevention is particularly important for conditions such as Alzheimer’s disease and other types of dementia that have no known cure. A prevention mindset requires a lifespan perspective, as physical activity and other healthy lifestyle behaviors such as good nutrition earlier in life impact health later in life.
Despite the Research, Americans Are Not Moving Enough
Even with so much data linking movement to better health outcomes, the U.S. is part of what has been described as a global pandemic of physical inactivity. Results of a national survey by the CDC published in 2022 found that 25.3% of Americans reported that outside of their regular job, they had not participated in any physical activity in the previous month, such as walking, golfing, or gardening. Rates of physical inactivity were even higher in Black and Hispanic adults, at 30% and 32%, respectively. Another survey highlighted rural-urban differences in the number of Americans who meet CDC physical activity guidelines that recommend ≥ 150 minutes per week of moderate-intensity aerobic exercise and ≥ 2 days per week of muscle-strengthening exercise. Respondents in large metropolitan areas were most active, yet only 27.8% met both aerobic and muscle strengthening guidelines. Even fewer people (16.1%) in non-metropolitan areas met the guidelines.
Why are so many Americans sedentary? The COVID-19 pandemic certainly exacerbated the problem; however, data from 2010 showed similar rates of physical inactivity, suggesting long-standing patterns of sedentary behavior in the country. Some of the barriers to physical activity are internal to the individual, such as lack of time, motivation, or energy. But other barriers are societal, at both the community and federal level. At the community level, barriers include transportation, affordability, lack of available programs, and limited access to high-quality facilities. Many of these barriers disproportionately impact communities of color and people with low income, who are more likely to live in environments that limit physical activity due to factors such as accessibility of parks, sidewalks, and recreation facilities; traffic; crime; and pollution. Action at the state and federal government level could address many of these environmental barriers, as well as financial barriers that limit access to exercise facilities and programs.
Physical Inactivity Takes a Toll on the Healthcare System and the Economy
Aside from a moral responsibility to promote the health of its citizens, the government has a financial stake in promoting movement in American society. According to recent analyses, inactive lifestyles cost the U.S. economy an estimated $28 billion each year due to medical expenses and lost productivity. Physical inactivity is directly related to the non-communicable diseases that place the highest burden on the economy, such as hypertension, heart disease, and obesity. In 2016, these types of modifiable risk factors comprised 27% of total healthcare spending. These costs are mostly driven by older adults, which highlights the increasing urgency to address physical inactivity as the population ages. Physical activity is also related to healthcare costs at an individual level, with savings ranging from 9-26.6% for physically active people, even after accounting for increased costs due to longevity and injuries related to physical activity. Analysis of 2012 data from the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey (MEPS) found that each year, people who met World Health Organization aerobic exercise guidelines, which correspond with CDC guidelines, paid on average $2,500 less in healthcare expenses related to heart disease alone compared to people who did not meet the recommended activity levels. Changes are needed at the federal, state, and local level to promote movement as medicine. If changes are not made in physical activity patterns by 2030, it is estimated that an additional $301.8 billion of direct healthcare costs will be incurred.
Government Agencies Can Play a Role in Better Promoting Physical Activity Programs
Promoting physical activity in the community requires education, resources, and removal of barriers in order for programs to have a broad reach to all citizens, including communities that are disproportionately impacted by the pandemic of physical inactivity. Integrated efforts from multiple agencies within the federal government is essential.
Past initiatives have met with varying levels of success. For example, Let’s Move!, a campaign initiated by First Lady Michelle Obama in 2010, sought to address the problem of childhood obesity by increasing physical activity and healthy eating, among other strategies. The Food and Drug Administration, Department of Agriculture, Department of Health and Human Services including the Centers for Disease Control and Prevention, and Department of Interior were among the federal agencies that collaborated with state and local government, schools, advocacy groups, community-based organizations, and private sector companies. The program helped improve the healthy food landscape, increased opportunities for children to be more physically active, and supported healthier lifestyles at the community level. However, overall rates of childhood obesity remained constant or even increased in some age brackets since the program started, and there is no evidence of an overall increase in physical activity level in children and adolescents since that time.
More recently, the U.S. Office of Disease Prevention and Health Promotion’s Healthy People 2030 campaign established data-driven national objectives to improve the health and well-being of Americans. The campaign was led by the Federal Interagency Workgroup, which includes representatives across several federal agencies including the U.S. Department of Health and Human Services, the U.S. Department of Agriculture, and the U.S. Department of Education. One of the campaign’s leading health indicators—a small subset of high-priority objectives—is increasing the number of adults who meet current minimum guidelines for aerobic physical activity and muscle-strengthening activity from 25.2% in 2020 to 29.7% by 2030. There are also movement-related objectives focused on children and adolescents as well as older adults, for example:
- Reducing the proportion of proportion of adults who do no physical activity in their free time
- Increasing the proportion of children, adolescents, and adults who do enough aerobic physical activity, muscle-strengthening activity, or both
- Increasing the proportion of child care centers where children aged 3 to 5 years do at least 60 minutes of physical activity a day
- Increasing the proportion of adolescents and adults who walk or bike to get places
- Increasing the proportion of children and adolescents who play sports
- Increasing the proportion of older adults with physical or cognitive health problems who get physical activity
- Increasing the proportion of worksites that offer an employee physical activity program
Unfortunately, there is currently no evidence of improvement in any of these objectives. All of the objectives related to physical activity with available follow-up data either show little or no detectable change, or they are getting worse.
To make progress towards the physical activity goals established by the Healthy People 2030 campaign, it will be important to identify where breakdowns in communication and implementation may have occurred, whether it be between federal agencies, between federal and local organizations, or between local organizations and citizens. Challenges brought on by the COVID-19 pandemic (e.g., less movement outside of the house for people who now work from home) will also need to be addressed, with the recognition that many of these challenges will likely persist for years to come. Critically, financial barriers should be reduced in a variety of ways, including more expansive coverage by the Centers for Medicare & Medicaid Services for exercise interventions as well as exercise for prevention. Policies that reflect a recognition of movement as medicine have the potential to improve the physical and mental health of Americans and address health inequities, all while boosting the health of the economy.
Towards a Well-Being Economy: Establishing an American Mental Wealth Observatory
Summary
Countries are facing dynamic, multidimensional, and interconnected crises. The pandemic, climate change, rising economic inequalities, food and energy insecurity, political polarization, increasing prevalence of youth mental and substance use disorders, and misinformation are converging, with enormous sociopolitical and economic consequences that are weakening democracies, corroding the social fabric of communities, and threatening social stability and national security. Globalization and digitalization are synchronizing, amplifying, and accelerating these crises globally by facilitating the rapid spread of disinformation through social media platforms, enabling the swift transmission of infectious diseases across borders, exacerbating environmental degradation through increased consumption and production, and intensifying economic inequalities as digital advancements reshape job markets and access to opportunities.
Systemic action is needed to address these interconnected threats to American well-being.
A pathway to addressing these issues lies in transitioning to a Well-Being Economy, one that better aligns and balances the interests of collective well-being and social prosperity with traditional economic and commercial interests. This paradigm shift encompasses a ‘Mental Wealth’ approach to national progress, recognizing that sustainable national prosperity encompasses more than just economic growth and instead elevates and integrates social prosperity and inclusivity with economic prosperity. To embark on this transformative journey, we propose establishing an American Mental Wealth Observatory, a translational research entity that will provide the capacity to quantify and track the nation’s Mental Wealth, generate the transdisciplinary science needed to empower decision makers to achieve multisystem resilience, social and economic stability, and sustainable, inclusive national prosperity.
Challenge and Opportunity
America is facing challenges that pose significant threats to economic security and social stability. Income and wealth inequalities have risen significantly over the last 40 years, with the top 10% of the population capturing 45.5% of the total income and 70.7% of the total wealth of the nation in 2020. Loneliness, isolation, and lack of connection are a public health crisis affecting nearly half of adults in the U.S. In addition to increasing the risk of premature mortality, loneliness is associated with a three-fold greater risk of dementia.
Gun-related suicides and homicides have risen sharply over the last decade. Mental disorders are highly prevalent. Currently, more than 32% of adults and 47% of young people (18–29 years) report experiencing symptoms of anxiety and depression. The COVID-19 pandemic compounded the burden, with a 25–30% upsurge in the prevalence of depressive and anxiety disorders. America is experiencing a social deterioration that threatens its continued prosperity, as evidenced by escalating hate crimes, racial tensions, conflicts, and deepening political polarization.
To reverse these alarming trends in America and globally, policymakers must first acknowledge that these problems are interconnected and cannot effectively be tackled in isolation. For example, despite the tireless efforts of prominent stakeholder groups and policymakers, the burden of mental disorders persists, with no substantial reduction in global burden since the 1990s. This lack of progress is evident even in high-income countries where investments in and access to mental health care have increased.
Strengthening or reforming mental health systems, developing more effective models of care, addressing workforce capacity challenges, leveraging technology for scalability, and advancing pharmaceuticals are all vital for enhancing recovery rates among individuals grappling with mental health and substance use issues. However, policymakers must also better understand the root causes of these challenges so we can reshape the economic and social environments that give rise to common mental disorders.
Understanding and Addressing the Root Causes
Prevention research and action often focus on understanding and addressing the social determinants of health and well-being. However, this approach lacks focus. For example, traditional analytic approaches have delivered an extensive array of social determinants of mental health and well-being, which are presented to policymakers as imperatives for investment. These include (but are not limited to):
- Adverse early life exposures (abuse and neglect)
- Substance misuse
- Domestic, family, and community violence
- Unemployment
- Poverty and inequality
- Poor education quality
- Homelessness
- Social disconnection
- Food insecurity
- Pollution
- Natural disasters and climate change
This practice is replicated across other public health and social challenges, such as obesity, child health and development, and specific infectious and chronic diseases. Long lists of social determinants lobbied for investment lead policymakers to conclude that nations simply can’t afford to invest sufficiently to solve these health and social challenges.
However, it Is likely that many of these determinants and challenges are merely symptoms of a more systemic problem. Therefore, treating the ongoing symptoms only perpetuates a cycle of temporary relief, diverts resources away from nurturing innovation, and impedes genuine progress.
To create environments that foster mental health and well-being, where children can thrive and fulfill their potential, where people can pursue meaningful vocation and feel connected and supported to give back to communities, and where Americans can live a healthy, active, and purposeful life, policymakers must recognize human flourishing and prosperity of nations depends on a delicate balance of interconnected systems.
The Rise of Gross Domestic Product: An Imperfect Measure for Assessing the Success and Wealth of Nations
To understand the roots of our current challenges, we need to look at the history of the foundational economic metric, gross domestic product (GDP). While the concept of GDP had been established decades earlier, it was during a 1960 meeting of the Organization for Economic Co-operation and Development that economic growth became a primary ambition of nations. In the shadow of two world wars and the Great Depression, member countries pledged to achieve the highest sustainable economic growth, employment, efficiency, and development of the world economy as their top priority (Articles 1 & 2).
GDP growth became the definitive measure of a government’s economic management and its people’s welfare. Over subsequent decades, economists and governments worldwide designed policies and implemented reforms aimed at maximizing economic efficiency and optimizing macroeconomic structures to ensure consistent GDP growth. The belief was that by optimizing the economic system, prosperity could be achieved for all, allowing governments to afford investments in other crucial areas. However, prioritizing the optimization of one system above all others can have unintended consequences, destabilizing interconnected systems and leading to a host of symptoms we currently recognize as the social determinants of health.
As a result of the relentless focus on optimizing processes, streamlining resources, and maximizing worker productivity and output, our health, social, political, and environmental systems are fragile and deteriorating. By neglecting the necessary buffers, redundancies, and adaptive capacities that foster resilience, organizations and nations have unwittingly left themselves exposed to shocks and disruptions. Americans face a multitude of interconnected crises, which will profoundly impact life expectancy, healthy development and aging, social stability, individual and collective well-being, and our very ability to respond resiliently to global threats. Prioritizing economic growth has led to neglect and destabilization of other vital systems critical to human flourishing.
Shifting Paradigms: Building the Nation’s Mental Wealth
The system of national accounts that underpins the calculation of GDP is a significant human achievement, providing a global standard for measuring economic activity. It has evolved over time to encompass a wider range of activities based on what is considered productive to an economy. As recently as 1993, finance was deemed “explicitly productive” and included in GDP. More recently, Biden-Harris Administration leaders have advanced guidance for accounting for ecosystem services in benefit-cost analyses for regulatory decision-making and a roadmap for natural capital inclusion in the nation’s economic accounting services. This shows the potential to expand what counts as beneficial to the American economy—and what should be measured as a part of economic growth.
While many alternative indices and indicators of well-being and national prosperity have been proposed, such as the genuine progress indicator, the vast majority of policy decisions and priorities remain focused on growing GDP. Further, these metrics often fail to recognize the inherent value of the system of national accounts that GDP is based on. To account for this, Mental Wealth is a measure that expands the inputs of GDP to include well-being indicators. In addition to economic production metrics, Mental Wealth includes both unpaid activities that contribute to the social fabric of nations and social investments that build community resilience. These unpaid activities (Figure 1, social contributions, Cs) include volunteering, caregiving, civic participation, environmental restoration, and stewardship, and are collectively called social production. Mental Wealth also includes the sum of investment in community infrastructure that enables engagement in socially productive activities (Figure 1, social investment, Is). This more holistic indicator of national prosperity provides an opportunity to shift policy priorities towards greater balance between the economy and broader societal goals and is a measure of the strength of a Well-Being Economy.
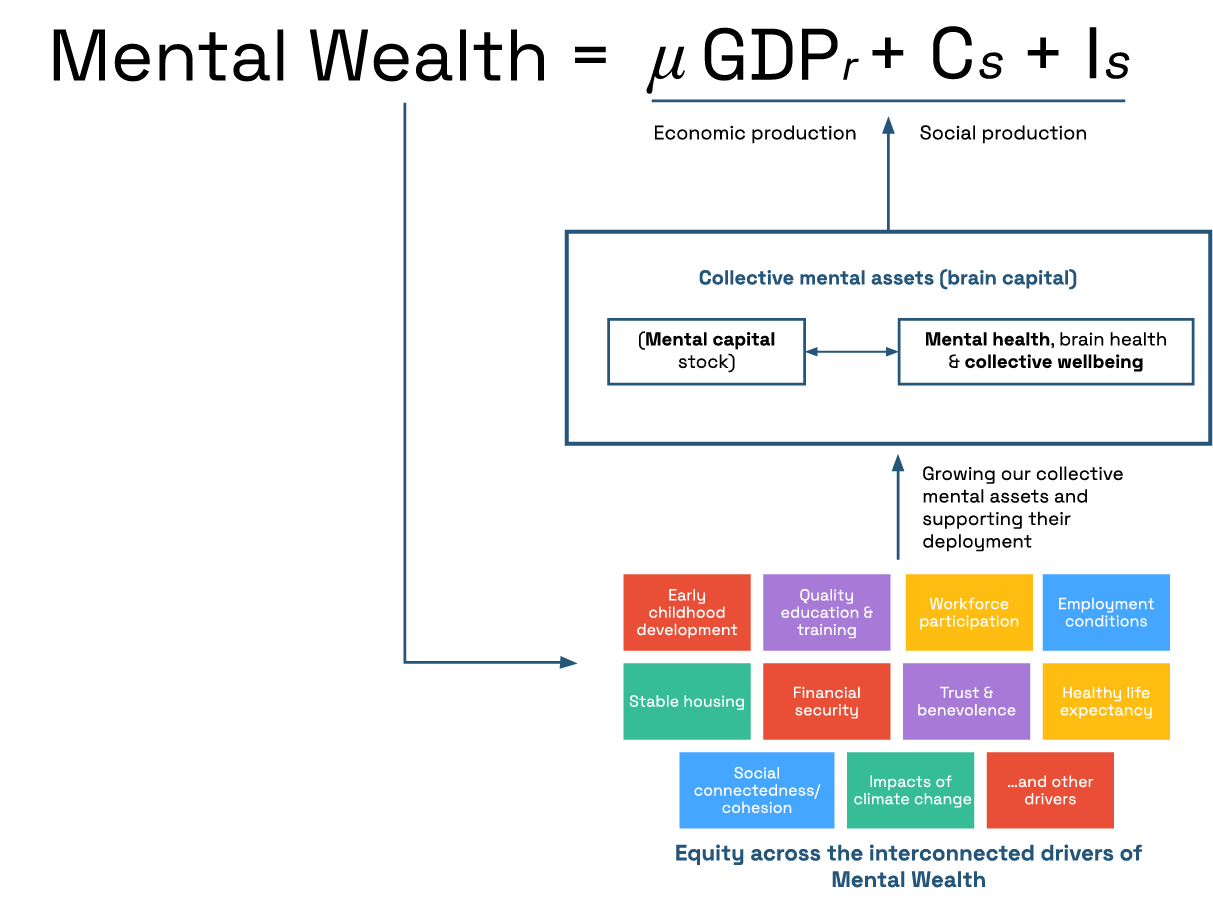
Mental wealth is a more comprehensive measure of national prosperity that monetizes the value generated by a nation’s economic and social productivity.
Valuing social production also promotes a more inclusive narrative of a contributing life, and it helps to rebalance societal focus from individual self-interest to collective responsibilities. A recent report suggests that, in 2021, Americans contributed more than $2.293 trillion in social production, equating to 9.8% of GDP that year. Yet social production is significantly underestimated due to data gaps. More data collection is needed to analyze the extent and trends of social production, estimate the nation’s Mental Wealth, and assess the impact of policies on the balance between social and economic production.
Unlocking Policy Insights through Systems Modeling and Simulation
Systems modeling plays a vital role in the transition to a Well-Being Economy by providing an understanding of the complex interdependencies between economic, social, environmental, and health systems, and guiding policy actions. Systems modeling brings together expertise in mathematics, biostatistics, social science, psychology, economics, and more, with disparate datasets and best available evidence across multiple disciplines, to better understand which policies across which sectors will deliver the greatest benefits to the economy and society in balance. Simulation allows policymakers to anticipate the impacts of different policies, identify strategic leverage points, assess trade-offs and synergies, and make more informed decisions in pursuit of a Well-Being Economy. Forecasting and future projections are a long-standing staple activity of infectious disease epidemiologists, business and economic strategists, and government agencies such as the National Oceanic and Atmospheric Administration, geared towards preparing the nation for the economic realities of climate change.
Plan of Action
An American Mental Wealth Observatory to Support Transition to a Well-Being Economy
Given the social deterioration that is threatening America’s resilience, stability, and sustainable economic prosperity, the federal government must systemically redress the imbalance by establishing a framework that privileges an inclusive, holistic, and balanced approach to development. The government should invest in an American Mental Wealth Observatory (Table 1) as critical infrastructure to guide this transition. The Observatory will report regularly on the strength of the Well-Being Economy as a part of economic reporting (see Table 1, Stream 1); generate the transdisciplinary science needed to inform systemic reforms and coordinated policies that optimize economic, environmental, health and social sectors in balance such as adding Mental Wealth to the system of national accounts (Streams 2–4); and engage in the communication and diplomacy needed to achieve national and international cooperation in transitioning to a Well-Being Economy (Streams 5–6).
This transformative endeavor demands the combined instruments of science, policy, politics, public resolve, social legislation, and international cooperation. It recognizes the interconnectedness of systems and the importance of a systemic and balanced approach to social and economic development in order to build equitable long-term resilience, a current federal interagency priority. The Observatory will make better use of available data from across multiple sectors to provide evidence-based analysis, guidance, and advice. The Observatory will bring together leading scientists (across disciplines of economics, social science, implementation science, psychology, mathematics, biostatistics, business, and complex systems science), policy experts, and industry partners through public-private partnerships to rapidly develop tools, technologies, and insights to inform policy and planning at national, state, and local levels. Importantly, the Observatory will also build coalitions between key cross-sectoral stakeholders and seek mandates for change at national and international levels.
The American Mental Wealth Observatory should be chartered by the National Science and Technology Council, building off the work of the White House Report on Mental Health Research Priorities. Federal partners should include, at a minimum, the Department of Health and Human Services (HHS) Office of the Assistant Secretary for Health (OASH), specifically the Office of the Surgeon General (OSG) and Office of Disease Prevention and Health Promotion (ODPHP); the Substance Abuse and Mental Health Services Administration (SAMHSA); the Office of Management and Budget; the Council of Economic Advisors (CEA); and the Department of Commerce (DOC), alongside strong research capacity provided by the National Science Foundation (NSF) and the National Institutes of Health (NIH).
Operationalizing the American Mental Wealth Observatory will require an annual investment of $12 million from diverse sources, including government appropriations, private foundations, and philanthropy. This funding would be used to implement a comprehensive range of priority initiatives spanning the six streams of activity (Table 2) coordinated by the American Mental Wealth Observatory leadership. Acknowledging the critical role of brain capital in upholding America’s prosperity and security, this investment offers considerable returns for the American people.
Conclusion
America stands at a pivotal moment, facing the aftermath of a pandemic, a pressing crisis in youth mental and substance use disorders, and a growing sense of disconnection and loneliness. The fragility of our health, social, environmental, and political systems has come into sharp focus, and global threats of climate change and generative AI loom large. There is a growing sense that the current path is unsustainable.
After six decades of optimizing the economic system for growth in GDP, Americans are reaching a tipping point where losses due to systemic fragility, disruption, instability, and civil unrest will outweigh the benefits. The United States government and private sector leaders must forge a new path. The models and approaches that guided us through the 20th century are ill-equipped to guide us through the challenges and threats of the 21st century.
This realization presents an extraordinary opportunity to transition to a Well-Being Economy and rebuild the Mental Wealth of the nations. An American Mental Wealth Observatory will provide the data and science capacity to help shape a new generation grounded in enlightened global citizenship, civic-mindedness, and human understanding and equipped with the cognitive, emotional, and social resources to address global challenges with unity, creativity, and resilience.
The University of Sydney’s Mental Wealth Initiative thanks the following organizations for their support in drafting this memo: FAS, OECD, Rice University’s Baker Institute for Public Policy, Boston University School of Public Health, the Brain Capital Alliance, and CSART.
Brain capital is a collective term for brain skills and brain health, which are fundamental drivers of economic and social prosperity. Brain capital comprises (1) brain skills, which includes the ability to think, feel, work together, be creative, and solve complex problems, and (2) brain health, which includes mental health, well-being, and neurological disorders that critically impact the ability to use brain skills effectively, for building and maintaining positive relationships with others, and for resilience against challenges and uncertainties.
Social production is the glue that holds society together. These unpaid social contributions foster community well-being, support our economic productivity, improve environmental wellbeing, and help make us more prosperous and resilient as a nation.
Social production includes volunteering and charity work, educating and caring for children, participating in community groups, and environmental restoration—basically any activity that contributes to the social fabric and community well-being.
Making the value of social production visible helps us track how economic policies are affecting social prosperity and allows governments to act to prevent an erosion of our social fabric. So instead of just measuring our economic well-being through GDP, measuring and reporting social production as well gives us a more holistic picture of our national welfare. The two combined (GDP plus social production) is what we call the overall Mental Wealth of the nation, which is a measure of the strength of a Well-Being Economy.
The Mental Wealth metric extends GDP to include not only the value generated by our economic productivity but also the value of this social productivity. In essence, it is a single measure of the strength of a Well-Being Economy. Without a Mental Wealth assessment, we won’t know how we are tracking overall in transitioning to such an economy.
Furthermore, GDP only includes the value created by those in the labor market. The exclusion of socially productive activities sends a signal that society does not value the contributions made by those not in the formal labor market. Privileging employment as a legitimate social role and indicator of societal integration leads to the structural and social marginalization of the unemployed, older adults, and the disabled, which in turn leads to lower social participation, intergenerational dependence, and the erosion of mental health and well-being.
Well-being frameworks are an important evolution in our journey to understand national prosperity and progress in more holistic terms. Dashboards of 50-80 indicators like those proposed in Australia, Scotland, New Zealand, Iceland, Wales, and Finland include things like health, education, housing, income and wealth distribution, life satisfaction, and more, which help track some important contributors to social well-being.
However, these sorts of dashboards are unlikely to compete with topline economic measures like GDP as a policy focus. Some indicators will go up, some will go down, some will remain steady, so dashboards lack the ability to provide a clear statement of overall progress to drive policy change.
We need an overarching measure. Measurement of the value of social production can be integrated into the system of national accounts so that we can regularly report on the nation’s overall economic and social well-being (or Mental Wealth). Mental Wealth provides a dynamic measure of the strength (and good management) of a Well-Being Economy. By adopting Mental Wealth as an overarching indicator, we also gain an improved understanding of the interdependence of a healthy economy and a healthy society.
Training for Safety and Success: Research & National Minimum Training Standards for Law Enforcement
Summary
Law enforcement is a highly visible profession where, without effective training, safety is at risk for both law enforcement officers and community members. Officers regularly respond to calls for service with uncertain risk factors and must balance the work with proactive activities to improve community well-being. Nationally, mandated training hours for new law enforcement officers are consistently less than those required for cosmetology licensure, with training quality and requirements varying significantly by state. Nearly three-quarters of states allow officers to work in a law enforcement function before completing the basic academy. Public trust and safety are placed in the hands of law enforcement officers, even if they lack the training, skills, and knowledge to be successful. Policing practices are regularly shaped by failures shown in national media, yet the shift in practices is rarely institutionalized in basic training practices.
To make communities safer and law enforcement officers more successful, the Biden-Harris Administration should fund research on the effectiveness of law enforcement training and create a national minimum standard for entry-level academy training to further support the Safer American Plan. The 2022 Executive Order on Advancing Effective, Accountable Policing and Criminal Justice Practices to Enhance Public Trust and Public Safety focuses on strengthening trust between communities and law enforcement officers, including training and equitable policing. The Department of Justice should oversee this research, and the Departments of Homeland Security, Labor, and Commerce can help create national standards and minimum training recommendations. Based on the findings and using pedagogical approaches for the most productive learning, minimum national training standards will be recommended by an interdisciplinary federal task force. Training can be used to compel change in law enforcement, improve community-police relations, and reduce liability while advancing community safety.
Challenge and Opportunity
Law enforcement actions have widespread implications due to the immense power and inherent risks associated with the position. The profession is plagued with complexity and unpredictability, further challenged by extensive discretionary capabilities and varied training requirements. Basic academy training is the foundational coursework for learning about laws and ethics, technical skills relating to actionable law enforcement functions, soft skill development, and honing critical thinking during stressful situations. However, more focus is placed on didactic portions with practical exercises than on cognitive, emotional, and social skills, which can be used to safely de-escalate situations. Even with these known training insufficiencies, academy training topics and hours are rarely updated. Training requirements and pedagogical approaches administered by peace officer standards and training or similar overseeing bodies generally require legislative updates to update curriculum standards, taking significant time and resources to enact change.
Back in 2015, President Obama highlighted the need for training and education in the 21st Century Taskforce on Policing, citing that law enforcement officers (LEOs) are required to be highly skilled in many operational areas to meet the wide variety of challenges and increasing expectations. The Biden-Harris Administration has vowed to advance effective, accountable policing through the Safer America Plan, noting that change at the local and state level requires congressional action. The Safer American Plan would provide funding for 100,000 additional LEOs, all of whom will require training to be effective in their role. Academy training requirements are not regularly collected or monitored at the federal level, and research is not routinely completed to show the efficacy of the training provided. The lack of data on law enforcement actions further complicates the training process, as the time spent during patrol is not regularly cataloged and reviewed to determine where officers spend most of their time. Data showing where officer time is spent can guide training decisions and adjust hours to provide skills for the most commonly utilized skill sets.
There is no national training standard for LEOs: state requirements vary from 1345 hours in the basic academy in Connecticut to 0 hours in Hawaii. The basic academy provides future LEOs foundational knowledge and skills in law, defensive tactics, report writing, first aid, communication, and other critical skills. The average length of basic training is 833 hours, with an average of 73 hours dedicated to firearm skills and 18 hours to de-escalation techniques. While firearm familiarization and skills are of utmost importance due to the fatal consequences of not understanding the weaponry and one’s ability, the discharge of a firearm occurs significantly less than de-escalation and other communication techniques. When not used regularly, skills become perishable, and the lack of regular training on topics like firearms and traffic stops can reduce an LEO’s efficiency, response time, and safety. The 2022 Executive Order on Advancing Effective, Accountable Policing mandates training federal LEOs with clear guidance on use-of-force standards and implicit bias, but these basic tenets of policing requirements are not extended to state and local law enforcement.
Thirty-seven states allow LEOs to work before they have completed a basic training academy. The time LEOs can work before receiving basic training ranges from 3 months in West Virginia to 24 months in Mississippi. There are obvious dangers to LEOs and the public by providing a uniform and firearm to an untrained person to interact with the community in a position of power. Figure 1 shows the ranges of when the basic academy is required of new LEOs.
With the basic academy averaging 833 hours, or about 21 weeks, it may seem like a sufficient timeframe to train new law enforcement officers. However, it commonly takes at least six months to master a new skill, with the academy requiring many new skills to be developed simultaneously. The minimum basic academy hour requirement in California is 664 hours, though the training is commonly over 1000 hours. By contrast, earning a cosmetology license in California has more extensive hour requirements than the basic police academy, with cosmetology and barber training requiring 1000 hours for state licensure. While injuries can occur in cosmetology, the profession is inherently safer for the practitioner and the client.
FBI Director Wray noted a 60% increase in murders of law enforcement officers in 2021, explicitly noting that violence against law enforcement officers does not receive as much attention as it should. Of the 245 LEOs who died in the line of duty in 2022, 74 were feloniously killed, up from 48 in 2019. In 2022, 1194 people were killed by LEOs, with 101 people being unarmed. Black people are disproportionately killed by LEOs, at nearly triple the population rate. The statistics of community members killed do not differentiate between legally justified uses of force and illegal actions, so a true picture of potential training concerns versus ethical violations cannot be determined.
Recognizing the insufficiencies of current LEO training raises opportunities for data-driven improvements. Research is needed to determine the efficacy of the basic academy training in each state, with comparisons made to provide an overall recommendation for minimum national standards. Innovation should be encouraged when developing future training standards, as the basic academy training has not embraced technology or newer learning techniques that may aid in practical decision-making and skill mastery.
Plan of Action
Training can be used to implement vital reforms in law enforcement, potentially saving lives. A multipronged, transparent approach is needed to determine the efficacy of current training before introducing innovation and minimum training standards. Multiple agencies will need to collaborate to complete the evaluation and create recommendations to incorporate inclusive views through multifaceted lenses and coordinate future actions. Transparency of the research and its goals, including making findings available on public-facing websites, is needed for accountability and to foster trust in the process of improving law enforcement. Additional detail of the proposed agencies and their roles is below.
Recommendation 1. Fund research for current LEO training and efficacy
Before overhauling training, data is needed to provide a baseline of training in each state, including its perceived efficacy by stakeholders. The DOJ should create and administer competitive grants to evaluate current training in every state/territory and complete surveys, interviews, and focus groups with stakeholders to determine the impact of training. Use-of-force incidents, accidents, LEO decertification, and other aspects of potential training deficiency should be examined for additional insight into effectiveness.
Research should also be conducted on fatal and accidental duty-related incidents to determine the human and other contributing factors. Data and trends gained from the research should be incorporated into minimum training standards to reduce future errors. Competitive grants can be provided to evaluate potential root causes of duty-related fatal and accidental deaths.
A key component of the research phase will be bringing the researchers together to discuss findings, regional and national trends, and recommendations. Creating a formal networking process will allow for best practices to be shared across all states/territories participating and made available to all LEO training commissions.
Recommendation 2. Spark innovation from adult learning experts and practitioners for LEO training
Through a competitive grant process, the DOJ’s Office of Justice Programs can advertise funding opportunities and outline the application process. Grants focusing on practitioners and adult learning experts in collaboration, potentially through practitioner-higher education partnerships, can assist in bringing the necessary experience from the field and adult learning. Curriculum designers should consider immersive or simulation training experiences and the use of technology in training. In addition, they should consider redesigning the rigid paramilitary format to encourage LEOs to utilize critical thinking skills, improve adaptability, and hone communication skills. Using Challenge.gov can also provide additional insights from the community.
Recommendation 3. Create national minimum standards for LEO basic academy training
Using the recommendations from the state law enforcement training researchers, the fatality factor researchers, practitioner and adult learner experts, FLETC, and DOL, a compilation of recommendations from NIST, DOJ, DHS, DOC, and DOL of national minimal standards should be completed. Requirements for academy instructors will also need to be established, including training program requirements and regular reviews of their performance and impact. NIST will use the information gathered, including contemporary training topics and a focus on adult learning techniques, and create a draft standard. The research teams and the public will have an opportunity to comment on the draft standards, then NIST will adjudicate the comments before sending the standards to an SDO for additional feedback for a quality, peer review.
The DOJ’s Office of Justice Programs will offer grants to all interested state LEO training bodies to adhere to the national minimum standard, with funding for planning, Implementation, and evaluation of the project. Grants should require a three-year timeline for implementation to ensure trainees receive training before their first day on the streets and the basic academy meets the minimum national requirements.
Recommendation 4. Evaluate curricula changes with environmental changes
Grant funding for the planning and implementation should extend an additional two years for the evaluation component. Evaluators chosen during the grant process can review how well training adheres to the national standards across all academies in the state, LEO feelings of preparedness upon graduation and quarterly after that for up to two years, and supervisor/administrator feedback on LEO performance after the academy. Deidentified records of unjustified use-of-force, decertification, and criminal actions can be reviewed for additional insight into the effectiveness of the basic academy training.
An overall program evaluation will be needed, including reviewing the state evaluations and the overall administration of the project. The grant can be open to one organization or multiple organizations with the selection and funding provided by DOJ’s Office of Justice Programs. Competitive grant funding for up to $5 million should be awarded for the six-to-eight-year evaluation.
Budget Proposal
A budget of $125 million is proposed to evaluate current LEO training, develop minimum requirements, and evaluate the implementation. The primary research of determining current LEO basic academy training and efficacy requires $500,000 for one researcher/research group per state/territory, totaling $28 million.
For the adult learning and practitioner component, competitive grants for up to 10 collaborations should receive up to $300,000 each, totaling $3 million. FLETC and DOL can be funded for their participation in the minimum standard creation at $1 million each, totaling $2 million.
Each state LEO training commission should be eligible to receive up to $2 million each to plan, implement, and evaluate the minimum training standards. If all states/territories participate, the funding will total $112 million.
An evaluation of the entire program will be conducted for $5 million for six to eight years of expected evaluative work. The final report will be provided to the DOJ to determine if performance metrics were met.
Conclusion
The national LEO training standard is meant to be the floor of training for states and does not remove the oversight of state peace officer training commissions. Every LEO should go through a basic academy and field training before serving the community to ensure they can be safe and effective in their roles. Developing innovating training techniques can help increase skills and understanding of vital topics while refining critical thinking skills in high-stress situations. Minimum training standards can improve safety for the public and first responders, reduce ethical and criminal violations by LEOs, and assist in repairing community-police relationships.
No. The 10th Amendment restricts the federal government from mandating standards, but federal grant funding can be restricted from states that do not meet the minimum training mandates. Precedence was made with DOJ’s Community Oriented Policing Services grants, which restrict federal funding if the agency’s use-of-force policy does not adhere to federal, state, and local laws.
States can update their training requirements at their will. States may be incentivized with federal grant funding, rather than waiting for unfunded and underresourced local attempts. Change involving many or all states can create pressure to conform to minimum requirements where there is currently little pressure with no financial incentives offered.
In December 2022, the House passed S.4003 Law Enforcement De-Escalation Training Act of 2022. The bill provides $34 million to the Department of Justice to fund scenario-based training for de-escalation and use-of-force for individuals experiencing a mental, suicidal, or behavioral crisis.
Stemming from the deaths of two unarmed Black men, HR 1280 and HR 1347 requested additional training and standards to reduce excessive force by LEOs. HR 1280 passed the House, and HR 1347 was introduced to the House with no actions since 2021.
LEO training in the United States is among the lowest in the world, with France training LEOs for 10 months or 1600 hours, Scotland’s basic training lasting for 92 weeks or 3680 hours, India for 2.5 years or 5400 hours, and Finland for three years or 6240 hours, with an additional year of field training.
Most states require continuing education or professional development. Hawaii has no LEO training requirements, and New Jersey law states agencies may provide in-service training without hourly requirements. Once minimum standards for basic training are implemented, national minimum mandatory annual continuing education or professional education can be developed.
The first recommendation requests funding to assess and determine the current efficacy of law enforcement training in every state. The multistage research would include interviews, surveys, and focus groups with stakeholders to determine training perceptions and impact, while a comparison is made using data from use-of-force incidents, officer decertification, accidents, fatal incidents, and other areas of potential training deficiency.
Transforming On-Demand Medical Oxygen Infrastructure to Improve Access and Mortality Rates
Summary
Despite the World Health Organization’s (WHO) designation of medical oxygen as an essential medicine in 2017, oxygen is still not consistently available in all care settings. Shortages in medical oxygen, which is essential for surgery, pneumonia, trauma, and other hypoxia conditions in vulnerable populations, existed prior to the COVID-19 pandemic and persist today. By one estimate, pre-pandemic, only 20% of patients in low- and middle-income countries (LMICs) who needed medical oxygen received it. The pandemic tremendously increased the need for oxygen, further compounding access issues as oxygen became an indispensable treatment. During the peak of the pandemic, dozens of countries faced severe oxygen shortages due to patient surges impacting an already fragile infrastructure.
The core driver of this challenge is not a lack of funding and international attention but rather a lack of infrastructure to buy oxygen, not just equipment. Despite organizations such as Unitaid, Bill & Melinda Gates Foundation, Clinton Health Access Initiative, UNICEF, WHO and U.S. Agency for International Development (USAID) prioritizing funding and provisions of medical oxygen, many countries still face critical shortages. Even fewer LMICs, such as Brazil, are truly oxygen self-sufficient. A broken and inequitable global oxygen delivery infrastructure inadvertently excludes low-income and rural area representation during the design phase. Furthermore, the current delivery infrastructure is composed of many individual funders and private and public stakeholders who do not work in a coordinated fashion because there is no global governing body to establish global policy, standards, and oversight; identify waste and redundancy; and ensure paths to self-sufficiency. As a result, LMICs are at the mercy of other nations and entities who may withhold oxygen during a crisis or fail to adequately distribute supply. It is time for aid organizations and governments to become more efficient and effective at solving this systemic problem by establishing global governance and investing in and enabling LMICs to become self-sufficient by establishing national infrastructure for oxygen generation, distribution, and delivery.
We propose transforming current interventions by centering the concept known as Oxygen as a Utility (OaaU), which fundamentally reimagines a country’s infrastructure for medical oxygen as a public utility supported by private investment and stable prices to create a functionable, equitable market for a necessary public health good. With the White House Covid Response Team shuttering in the coming months, USAID’s Bureau for Global Health has a unique opportunity to take a global leadership role in spearheading the development of an accessible, affordable oxygen marketplace. USAID should convene a global public-private partnership and governing coalition called the Universal Oxygen Coalition (UOC), pilot the OaaU model in at least two target LMICs (Tanzania and Uttar Pradesh, India), and launch a Medical Oxygen Grand Challenge to enable necessary technological and infrastructure innovation.
Challenge and Opportunity
There is no medical substitute for oxygen, which is used to treat a wide range of acute respiratory distress syndromes, such as pneumonia and pneumothorax in newborns, and noncommunicable diseases, such as asthma, heart failure, and COVID-19. Pneumonia alone is the world’s biggest infectious killer of adults and children, claiming the lives of 2.5 million people, including 740,180 children, in 2019. The COVID-19 pandemic compounded the demand for oxygen, and exposed the lack thereof, with increased death tolls in countries around the world as a result.
For every COVID-19 patient who needs oxygen, there are at least five other patients who also need it, including the 7.2 million children with pneumonia who enter LMIC hospitals each year. [Ehsanur et al, 2021]. Where it is available, there are often improperly balanced oxygen distribution networks, such as high-density areas being overstocked while rural areas or tertiary care settings go underserved. Only 10% of hospitals in LMICs have access to pulse oximetry and oxygen therapy, and those better-resourced hospitals tend to be in larger cities closer to existing oxygen delivery providers.
This widespread lack of access to medical oxygen in LMICs threatens health outcomes and well-being, particularly for rural and low-income populations. The primary obstacle to equitable oxygen access is lack of the necessary digital infrastructure in-country. Digital infrastructure provides insights that enable health system managers and policymakers to effectively establish policy, manage the supply of oxygen to meet needs, and coordinate work across a complex supply chain composed of various independent providers. Until replicable and affordable digital infrastructure is established, LMICs will not have the necessary resources to manage a national oxygen delivery system, forecast demand, plan for adequate oxygen production and procurement, safeguard fair distribution, and ensure sustainable consumption.
Oxygen can be delivered in a number of forms—via concentrators, cylinders, plants, or liquid—and the global marketplace encompasses many manufacturers and distributors selling in multiple nations. Most oxygen providers are for-profit organizations, which are not commercially incentivized to collaborate to achieve equal oxygen access, despite good intentions. Many of these same manufacturers also sell medical devices to regulate or deliver oxygen to patients, yet maintaining the equipment across a distributed network remains a challenge. These devices are complex and costly, and there are often few trained experts in-country to repair broken devices. Instead of recycling or repairing devices, healthcare providers are often forced to discard broken equipment and purchase new ones, contributing to greater landfill waste and compounding health concerns for those who live nearby.
Common contributing causes for fragmented oxygen delivery systems in LMICs include:
- No national digital infrastructure to connect, track, and monitor medical oxygen supply and utilization, like an electrical utility to forecast demand and ensure reliable service delivery.
- No centralized way to monitor manufacturers, distributors, and the various delivery providers to ensure coordination and compliance with local policy.
- In many cases, no established local policy for oxygen and healthcare regulation or no means to enforce local policy.
- Lack of purchasing options for healthcare providers, who are often forced to buy whichever oxygen devices are available versus the type of source oxygen that best fits their needs (i.e., concentrator or liquid) due to cumbersome tender systems and lack of coordination across markets.
- Lack of trained experts to maintain and repair devices, including limited national standardized certification programs, resulting in the premature disposal of costly medical devices contributing to waste issues. Further, lack of maintenance fuels the vicious cycle of LMICs requiring more regular funding to buy oxygen devices, which can increase reliance on third parties to sustain oxygen needs rather than domestic demand and marketplaces.
Medical oxygen investment is a unique opportunity to achieve global health outcomes and localization policy objectives. USAID invested $50 million to expand medical oxygen access through its global COVID-19 response for LMIC partners, but this investment only scratches the surface of what is needed to deliver self-sustainment. In response to oxygen shortages during the peaks of the pandemic, the WHO, UNICEF, the World Bank, and other donors shipped hundreds of thousands of oxygen concentrators to help LMICs deal with the rise in oxygen needs. This influx of resources addressed the interim need but did not solve the persisting healthcare system and underlying oxygen infrastructure problems. In 2021, the World Bank made emergency loans available to LMICs to help them shore up production and infrastructure capabilities, but not enough countries applied for these loans, as the barriers to solve these infrastructure issues are complex, difficult to identify without proper data and digital infrastructure to identify supply chain gaps, and hard to solve with a single cash loan.
Despite heavy attention to the issue of oxygen access in LMICs, current spending does not go far enough to set up sustainable oxygen systems in LMICs. Major access and equity gaps still persist. In short, providing funding alone without a cohesive, integrated industrial strategy cannot solve the root problem of medical oxygen inequality.
USAID recently announced an expanded commitment in Africa and Asia to expand medical oxygen access, including market-shaping activities and partnerships. Since the pandemic began, USAID has directed $112 million in funding for medical oxygen to 50 countries and is the largest donor to The Global Fund, which has provided the largest international sums of money (more than $600 million) to increase medical oxygen access in over 80 countries. In response to the pandemic’s impacts on LMICs, the ACT-Accelerator (ACT-A) Oxygen Emergency Taskforce, co-chaired by Unitaid and the Wellcome Trust, has provided $700 million worth of oxygen supplies to over 75 countries and catalyzed large oxygen suppliers and NGO leaders to support LMICs and national healthcare ministries. This task force has brought together industry, philanthropy, NGO, and academic leaders. While USAID is not a direct partner, The Global Fund is a primary donor to the task force.
Without a sea change in policy, however, LMICs will continue to lack the support required to fully diagnosis national oxygen supply delivery system bottlenecks and barriers, establish national regulation policies, deploy digital infrastructures, change procurement approaches, enable necessary governance changes, and train in-country experts to ensure a sustained, equitable oxygen supply chain. To help LMICs become self-sufficient, we need to shift away from offering a piecemeal approach (donating money and oxygen supplies) to a holistic approach that includes access to a group of experts , funding for oxygen digital infrastructure systems, aid to develop national policy and governance mechanisms, and support for establishing specialty training and certification programs so that LMICs can self-manage their own medical oxygen supply chain. Such a development policy initiative relies on the Oxygen as a Utility framework, which focuses on creating a functional, equitable market for medical oxygen as a necessary public good. When achieved successfully, OaaU facilitates one fair rate for end-to-end distribution within a country, like other public utilities such as water and electricity.
A fully realized OaaU model within a national economy would integrate and streamline most aspects of oxygen delivery, from production to distribution of both the oxygen and the devices that dispense it, to training of staff on when to administer oxygen, how to use equipment, and equipment maintenance. This proposed new model coordinates industry partners, funders, and country leaders to focus on end-to-end medical oxygen delivery as an affordable, accessible utility rather than an in-kind development good. OaaU centers predictability, affordability, and efficiency for each stakeholder involved in creating sustainable LMIC medical oxygen supply chains. At its core, OaaU is about increasing both access and reliability by providing all types of oxygen at negotiated, market-wide, affordable, and predictable prices through industry partners and local players. This new business model would be sustainable by charging subscription and pay-per-use fees to serve the investment by private sector providers, each negotiated by Ministries of Health to empower them to manage their own country’s oxygen needs. This new model will incorporate each stakeholder in an LMIC’s healthcare system and facilitate an open, market-based negotiation to achieve affordable, self-sufficient medical oxygen supply chains.
Initial investment is needed to create a permanent oxygen infrastructure in each LMIC to digitally transform the tender system from an equipment and service or in-kind aid model to buying oxygen as a utility model. An industry business model transformation of this scale will require multistakeholder effort to include in-country coordination. The current oxygen delivery infrastructure is composed of many individual funders and private and public stakeholders who do not work in a coordinated fashion. At this critical juncture for medical oxygen provision, USAID’s convening power, donor support, and expertise should be leveraged to better direct this spending to create innovative opportunities. The Universal Oxygen Coalition would establish global policy, standards, and oversight; identify waste and redundancy; and ensure viable paths to oxygen self-sufficiency in LMICs. The UOC will act similarly to electric cooperatives, which aggregate supplies to meet electricity demand, ensuring every patient has access to oxygen, on demand, at the point of care, no matter where in the world they live.
Plan of Action
To steward and catalyze OaaU, USAID should leverage its global platform to convene funders, suppliers, manufacturers, distributors, health systems, financial partners, philanthropy, and NGOs and launch a call to action to mobilize resources and bring attention to medical oxygen inequality. USAID’s Bureau for Global Health, along with the its Private Sector Engagement Points of Contact, and the State Department’s Office of Global Partnerships should spearhead the UOC coalition. Using USAID’s Private Sector Engagement Strategy and EDGE fund as a model, USAID can serve as a connector, catalyzer, and lead implementer in reforming the global medical oxygen marketplace. The Bureau for Global Health should organize the initial summit, calls to action, and burgeoning UOC coalition because of its expertise and connections in the field. We anticipate that the UOC would require staff time and resources, which could be funded by a combination of private and philanthropic funding from UOC members in addition to some USAID resources.
To achieve the UOC vision, multiple sources of funding could be leveraged in addition to Congressional appropriation. In 2022, State Department and USAID funding for global health programs, through the Global Health Programs (GHP) account, which represents the bulk of global health assistance, totaled $9.8 billion, an increase of $634 million above the FY21 enacted level. In combination with USAID’s leading investments in The Global Fund, USAID could deploy existing authorities and funding from Development Innovation Ventures’ (DIV) and leverage Grand Challenge models like Saving Lives at Birth to create innovation incentive awards already authorized by Congress, or the newly announced EDGE Fund focused on flexible public-private sector partnerships to direct resources toward achieving equitable oxygen access for all. These transformative investments would also serve established USAID policy priorities like localization. UOC would work with USAID and the Every Breath Counts Initiative to reimagine this persistent problem by bringing essential players—health systems, oxygen suppliers, manufacturers and/or distributors, and financial partners—into a unified holistic approach to ensure reliable oxygen provision and sustainable infrastructure support.
Recommendation 1. USAID’s Bureau for Global Health should convene the Universal Oxygen Coalition Summit to issue an OaaU co-financing call to action and establish a global governing body.
The Bureau for Global Health should organize the summit, convene the UOC coalition, and issue calls to action to fund country pilots of OaaU. The UOC coalition should bring together LMIC governments; local, regional, and global private-sector medical oxygen providers; local service and maintenance companies; equipment manufacturers and distributors; health systems; private and development finance; philanthropy organizations; the global health NGO community; Ministries of Health; and in-country faith-based organizations.
Once fully established, the UOC would invite industry coalition members to join to ensure equal and fair representation across the medical oxygen delivery care continuum. Potential industry members include Air Liquide, Linde, Philips, CHART, Praxair, Gulf Cryo, Air Products, International Futures, AFROX, SAROS, and GCE. Public and multilateral institutions should include the World Bank, World Health Organization, UNICEF, USAID country missions and leaders from the Bureau for Global Health, and selected country Ministries of Health. Funders such as Rockefeller Foundation, Unitaid, Bill & Melinda Gates Foundation, Clinton Health Access Initiative, and Wellcome Trust, as well as leading social enterprises and experts in the oxygen field such as Hewatele and PATH, should also be included.
UOC members would engage and interact with USAID through its Private Sector Engagement Points of Contact, which are within each regional and technical bureau. USAID should designate at least two points of contact from a regional and technical bureau, respectively, to lead engagement with UOC members and country-level partners. While dedicated funds to support the UOC and its management would be required in the long term either from Congress or private finance, USAID may be able to deploy staff from existing budgets to support the initial stand-up process of the coalition.
Progress and commitments already exist to launch the UOC, with Rockefeller Philanthropy Advisors planning to bring fiscal sponsorship as well as strategy and planning for the formation of the global coalition to the UOC with PATH providing additional strategic and technical functions for partners. The purpose of the UOC through its fiscal sponsor is to act as the global governing body by establishing global policy, standards, oversight controls, funding coordination, identifying waste & redundancy, setting priorities, acting as advisor and intermediary when needed to ensure LMIC paths to self-sufficiency are available. UOC would oversee and manage country selection, raising funding, and coordination with local Ministries of Health, funders, and private sector providers.
Other responsibilities of the UOC may include:
- Issue feasibility studies to assess technology gaps in target countries. This research would inform future challenges, contracts, prioritization, design, and focus.
- Advise LMICs on identifying barriers and knowing best next steps.
- Establish an official framework of best practices for OaaU that includes core metrics of success and replicable models.
The first UOC Summit will issue a call to action to make new, significant commitments from development banks, philanthropies, and aid agencies to co-finance OaaU pilot programs, build buy-in within target LMICs, and engage in market-shaping activities and infrastructure investments in the medical oxygen supply chain. The Summit could occur on the sidelines of the Global COVID-19 Summit or the United Nations General Assembly. Summit activities and outcomes should include:
- Announce the launch and secure financial commitments from public and private funds for piloting OaaU in at least one national context.
- Identify and prioritize criteria for selecting pilot locations (regions or nations) for OaaU and select the initial country(s) for holistic oxygen self-sufficiency investment.
- Create the UOC Board representing manufacturers, global health experts, LMIC leaders, funders, multilateral institutions, and health providers who are empowered to identify geographic areas most in need of oxygen investment, issue market-specific grants and open innovation competitions, and leverage pooled public and private funds.
- Research, prioritize, and select at least two models of OaaU within a national marketplace to focus attention of all stakeholders on fixing the oxygen marketplace.
Recommendation 2. The UOC should establish country prioritization based on need and readiness and direct raised funds toward pilot programs.
USAID should co-finance an OaaU pilot model through investments in domestic supply chain streamlining and leverage matched funds from development bank, private, and philanthropic dollars. This fund should be used to invest in the development of a holistic oxygen ecosystem starting in Tanzania and in Uttar Pradesh, India, so that these regions are prepared to deliver reliable oxygen supply, catalyzing broad demand, business activity, and economic development.
The objective is to deliver a replicable global reference model for streamlining the supply chain and logistics, eventually leading to equitable oxygen catering to the healthcare needs that can be rolled out in other LMICs and improve lives for the deprived. The above sites are prioritized based on their readiness and need as determined by the 2020 PATH Market Research Study supported by the Bill and Melinda Gates Foundation. We estimate that $495 million for the pilots in both nations would provide oxygen for 270 million people, which equates to less than $2 per person. The UOC should:
- Invest in local providers: This will generate economic development and high-paying jobs in-country and throughout the supply chain.
- Spur localized innovation and digital transformation: Foster locally driven innovation by in-country and regional systems integrators, especially in digital transformation of oxygen generation, distribution, and delivery. Solutions should include new digital tools for aggregation of supply and demand and real-time command and control to radically improve access to medical oxygen on demand.
- Create an in-country deployment coalition for each pilot country: Because oxygen marketplaces are unique to each context, having a market-based deployment coalition in each country that involves private, public, and social sector partners is critical to coordinating the deployment of resources and maintaining implementation efforts. The deployment coalition could be operated out of or supervised by a USAID Country Mission.
- Provide pilot model funding to enable Ministries of Health and the deployment coalition to streamline and fix supply chains.
- Issue calls to action for interested parties and stakeholders to submit plans to address both the immediate medical oxygen needs in the country of choice and the long-term infrastructure barriers. These plans could help inform strategy for deploying resources and making oxygen infrastructure investments.
This effort will result in a sustainable oxygen grid in LMICs to produce revenue via subscription and pay-per-use model, reducing the need for aid organization or donor procurement investment on an annual basis. To create the conditions for OaaU, the UOC will need to make a one-time investment to create infrastructure that can provide the volume of oxygen a country needs to become oxygen self-sufficient. This investment should be backed by the World Bank via volume usage guarantees similar to volume usage guarantees for electricity per country. The result will shift the paradigm from buying equipment to buying oxygen.
Recommendation 3. The UOC and partner agencies should launch the Oxygen Access Grand Challenge to invest in innovations to reduce costs, improve maintenance, and enhance supply chain competition in target countries.
We envision the creation of a replicable solution for a self-sustaining infrastructure that can then serve as a global reference model for how best to streamline the oxygen supply chain through improved infrastructure, digital transformation, and logistics coordination. Open innovation would be well-suited to priming this potential market for digital and infrastructure tools that do not yet exist. UOC should aim to catalyze a more inclusive, dynamic, and sustainable oxygen ecosystem of public- and private-sector stakeholders.
The Grand Challenge platform could leverage philanthropic and private sector resources and investment. However, we also recommend that USAID deploy some capital ($20 million over four years) for the prize purse focused on outcomes-based technologies that could be deployed in LMICs and new ideas from a diverse global pool of applicants. We recommend the Challenge focus on the creation of digital public goods that will be the digital “command and control” backbone of a OaaU in-country. This would allow a country’s government and healthcare system to know their own status of oxygen supply per a country grid and which clinic used how much oxygen in real time and bill accordingly. Such tools do not yet exist at affordable, accessible levels in LMICs. However, USAID and its UOC partners should scope and validate the challenge’s core criteria and problems, as they may differ depending on the target countries selected.
Activities to support the Challenge should include:
- Assessing technology and cost gaps in target partner countries in healthcare infrastructure, with a particular focus on supply chains and oxygen provision. This research would inform the Challenge design and focus.
- Creating partnerships with LMICs to implement promising innovations in pilots and secure advanced market commitments from healthcare ministries, the private sector, and multilateral or private financing to ensure viable pathways to scale for solutions.
- Establishing an official framework of best practices for OaaU that includes core metrics of success and replicable models that interested healthcare ministries could use to develop a system in their own nations.
Conclusion
USAID can play a catalytic role in spearheading the creation and sustainment of medical oxygen through a public utility model. Investing in new digital tools for aggregation of supply and demand and real-time command and control to radically improve access to medical oxygen on demand in LMICs can unlock better health outcomes and improve health system performance. By piloting the OaaU model, USAID can prove the sustainability and scalability of a solution that can be a global reference model for streamlining medical oxygen supply chain and logistics. USAID and its partners can begin to create sustained change and truly equitable oxygen access. Through enhancing existing public-private partnerships, USAID can also cement a resilient medical oxygen system better prepared for the next pandemic and better equipped to deliver improved health outcomes.
References
- Pneumonia in Children Statistics – UNICEF DATA
- Ann Danaiya Usher (2021). Medical oxygen crisis: a belated COVID-19 response. The
Lancet, World Report. - Lam F., Stegmuller A.,Chouz V.B., Grahma H.R. (2021). Oxygen systems strengthening as
an intervention to prevent childhood deaths due to pneumonia in low-resource settings:
systematic review, meta-analysis and cost-effectiveness. BMJ Global Health Journals - AD Usher: Medical Oxygen crisis: a belated COVID-19 response (2021) The Lancet Global
Health. - Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS et al.
(2013) Global and regional burden of hospital admissions for severe acute lower respiratory
infections in young children in 2010: a systematic analysis. Lancet 381: 1380–90.
10.1016/S0140-6736(12)61901-1 - Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE et al. (2012) Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379: 2151–61. 10.1016/S0140-6736(12)60560-1
- UNEP report. Africa waste management outlook. (2018)
- T Duke, SM Gramham, NN Cherian, AS Ginsburg, M English, S Howie, D Peel, PM Enarson,
IH Wilson, and W Were, the Union Oxygen Systems Working Group (2010) Oxygen is an
essential medicine: A call for international action. - Unitaid press release. COVID-19 emergency impacting more than half a million people in low-
and middle-income countries every day, as demand surges (2021)
The OaaU approach integrates and streamlines most aspects of oxygen delivery, just as integrated power grids grew into public utilities through government investment and public-private partnerships built on technological development to manage them. With an OaaU approach, investments would be made in oxygen digital grid design, build, interoperable connectivity across markets, staff training, demand forecasting and development of a longitudinal sustainable plan. Through this model, an increased number of oxygen suppliers would compete through auctions designed to drive down cost. Governments would receive a lower fixed price in exchange for offering a firm commitment to purchase a pre-established amount of oxygen, services, and equipment to provide oxygen over a long-time horizon. Financial partners guarantee the value of these commitments to reduce the risk that countries will default on their payments, seeking to encourage the increased competition that turns the wheels of this new mechanism. Providing a higher-quality, lower-cost means of obtaining medical oxygen would be a relief for LMICs. Additionally, we would anticipate the government to play a greater role in regulation and oversight which would provide price stability, affordability, and adequate supply for markets—just like how electricity is regulated.
First, oxygen is a complex product that can be generated by concentrators, cylinders, plants, and in liquid oxygen form. For a country to become oxygen self-sufficient, it needs all types of oxygen, and each country has its own unique combination of needs based on healthcare systems, population needs, and existing physical infrastructure. If a country has an excellent transportation system, then delivery of oxygen is the better choice. But if a country has a more rural population and no major highways, then delivery is not a feasible solution.
The oxygen market is competitive and consists of many manufacturers, each of which bring added variations to the way oxygen is delivered. While WHO-UNICEF published minimal technical specifications and guidance for oxygen therapy devices in 2019, there remains variation in how these devices are delivered and the type of data produced in the process. Additionally, oxygen delivery requires an entire system to ensure it safely reaches patients. In most cases, these systems are decentralized and independently run, which further contributes to service and performance variation. Due to layers of complexity, access to oxygen includes multiple challenges in availability, quality, affordability, management, supply, human resources capacity, and safety. National oversight through a digital oxygen utility infrastructure that requires the coordination and participation of the various oxygen delivery stakeholders would address oxygen access issues and enable country self-sustainment.
Given that oxygen provides areturn of US $50 per disability-adjusted life year, medical oxygen investment is a meaningful opportunity for development banks, foreign assistance agencies, and impact investors. The OaaU business model transformation will be a major step toward oxygen availability in the form of oxygen on-demand in LMICs. Reliable, affordable medical oxygen can strengthen the healthcare infrastructure and improve health outcomes. Recent estimates indicate every year about 120–156 million cases of acute lower respiratory infections occur globally in children under five, with approximately 1.4 million resulting in death. More than 95% of these deaths occur in low- and middle-income countries (Nair, 2013; Lui, 2012).
Unlike prior approaches, OaaU is a business model transformation from partial solutions to integrated solutions with all types of oxygen, just like the electricity sector transformed into an integrated grid of all types of electricity supply. From there, the medical facilities will buy oxygen, not equipment—just like you buy amounts of electricity, not a nuclear power plant.
A Bipartisan Health Agenda to Unite America: Innovative Ideas to Strengthen American Wellbeing
As the COVID-19 pandemic has clearly shown – American health is crucial to the health of our nation. Yet American health is under threat from all angles, from escalating chronic deadly diseases like cancer to rising mental health challenges and the growing overdose epidemic. All of these threats contribute to the United States ranking 31st in life expectancy at birth, one of the lowest in the developed world, despite having the highest health spending per capita.
At the State of the Union, the Biden Administration presented a bipartisan platform dedicated to securing the health and wellbeing of the American people, from our Veterans to our youth. An agenda is a first step – unified action on public health comes next. Evidence-based science policy can bring us closer to a healthier future. Since 2020, policy entrepreneurs have developed innovative implementation-ready policy proposals through the Day One Project (D1P) to tackle some of the biggest societal problems. Here are a few that speak to the current moment:
To combat cancer…
With the median monthly cost of cancer drugs topping $10,000, many families cannot afford the costs of caring for their loved ones. Yet, there are 1,100 FDA-approved off-patent generics that could be used for treating cancer, at a fraction of the cost. Congress should appropriate $100 million into Phase III clinical trials of off-patent generics for treating a variety of cancers. This funding can go towards the National Cancer Institute and be implemented through an open-source pharmaceutical R&D framework through accelerated progress towards accessible and affordable cures.
Environmental hazards are a growing driver of cancers, and disproportionately impact rural and disadvantaged communities. Air pollution has been linked to lung cancer, the most deadly cancer for both men and women in the US. An interagency collaboration led by National Oceanic and Atmospheric Administration and leveraging funds from the Inflation Reduction Act could deploy a network on low-cost, real-time, ground-based sensors in all 300 US cities with a population above 100,000 to track particulate matter rates. Connecting this data to relevant providers in these cities, such as federally-qualified community health centers, could inform physicians of high-risk sites to target early screening interventions. Further, materials composing American homes, from housing materials to pipe materials, and even water running in the faucets, have been identified as possible sources of carcinogens. The Biden Administration should launch the President’s Task Force on Healthy Housing and Water for Cancer Prevention to coordinate research, develop the statistical database, and prepare for regulatory actions.
Finally, innovations in primary care can also catch cancer at earlier stages in disease progression. Yet, many rural and disadvantaged communities lack access to primary care. The NIH’s $23 million investment investigating telehealth for cancer care will develop the best care strategies – but labor-market, technical, financial/regulatory barriers, and data barriers will remain for scaling to the broad population. The Biden Administration and Congress will need to collaborate to unlock barriers to delivering healthcare services directly to the American home, through reforming licensure, expanding broadband access, investing in new mobile healthcare devices, expanding Medicare and Medicaid reimbursements, and ensuring data interoperability.
To strengthen mental health…
Digital mental health technologies have enormous potential to combat the growing mental health crisis, as evidenced by the Administration’s plan on mental health research and development. Yet more work remains to build a national infrastructure for successful implementation of digital mental health services. The vast majority of digital mental health technologies are unregulated, as existing FDA standards fail to cover these emerging technologies because many do not make treatment claims. Congress should authorize Health and Human Services (HHS) to develop standards for digital mental health products to ensure clinical effectiveness, data safety, and mitigate risk. Technologies that meet these standards should then be reimbursable through Medicare and Medicaid, which will require further congressional action. Finally, the Substance Abuse and Mental Health Services Administration (SAMHSA) should create a National Center for Digital Mental Health to maintain a database of approved digital products, provide training to providers, and ensure compliance of developers with national standards.
Knowing that tech platforms can be harmful to the youth’s wellbeing, the Congress and the Administration can take several steps to protect children’s privacy. Congress can expand the technological expertise at the Department of Education (ED) to protect children’s privacy and security in schools as well as appropriate $160 million funding to the Federal Trade Commission (FTC) to expand Children’s Online Protection Privacy Act (COPPA) enforcement and further investigate technology companies extracting children’s data. The Administration can commission a task force to identify ways to protect children’s data through existing legislation such as the Family Educational Rights and Privacy Act and COPPA.
To tackle the opioid crisis…
The opioid crisis is claiming thousands of lives every year, and there is bipartisan consensus on action. The Centers for Medicare and Medicaid (CMS) has sought strategies to prevent opioid use disorders – which will require reforms to the insurance reimbursement model which less generously covers preventative services. The Biden Administration should pilot a multidisciplinary study group to implement payment for prevention, using opioid use disorders as the test case. Following the guidance of the study group, CMS should provide guidelines to contracts between states and managed care organizations (MCOs) and between MCOs and providers and provide necessary technical assistance to implement these guidelines.
To deliver on care for Veterans…
Five million veterans live in rural areas, and of those, 45% lack access to reliable broadband internet, reducing access to vital health services. To ensure Veterans remain connected to healthcare services wherever they are, the Veterans Health Administration (VHA) should partner with the Postal Service and/or Department of Agriculture to pilot telehealth hubs in rural communities using existing FY23 appropriations for telehealth. An initial focus of care delivery could be on digital mental health and suicide prevention. Going forward, care delivery innovations like this one, if successful, can inspire new policies for the broader population, if the VHA’s health policy mission is expanded. VHA should be added to strategic interagency health policy coalitions such as the ACA interagency working group on healthcare quality and Healthy People 2030 to share data, develop innovative projects, and evaluate progress.
There’s more work to be done to build a healthier future for all Americans – these ideas can be jumping off points for Executive and Congressional action. FAS will continue to develop and surface evidence-based policies that can make a difference, and submissions to the Day One project are always welcome.
Enabling Faster Funding Timelines in the National Institutes of Health
Summary
The National Institutes of Health (NIH) funds some of the world’s most innovative biomedical research, but rising administrative burden and extended wait times—even in crisis—have shown that its funding system is in desperate need of modernization. Examples of promising alternative models exist: in the last two years, private “fast science funding” initiatives such as Fast Grants and Impetus Grants have delivered breakthroughs in responding to the coronavirus pandemic and aging research on days to one-month timelines, significantly faster than the yearly NIH funding cycles. In response to the COVID-19 pandemic the NIH implemented a temporary fast funding program called RADx, indicating a willingness to adopt such practices during acute crises. Research on other critical health challenges like aging, the opioid epidemic, and pandemic preparedness deserves similar urgency. We therefore believe it is critical that the NIH formalize and expand its institutional capacity for rapid funding of high-potential research.
Using the learnings of these fast funding programs, this memo proposes actions that the NIH could take to accelerate research outcomes and reduce administrative burden. Specifically, the NIH director should consider pursuing one of the following approaches to integrate faster funding mechanisms into its extramural research programs:
- Reform the existing R21 grant mechanism to bring it more in line with its own goals of funding high-reward, rapid-turnaround research; and
- Direct NIH institutes and centers to independently develop and deploy new research programs with faster funding timelines.
Future efforts by the NIH and other federal policymakers to respond to crises like the COVID-19 pandemic would also benefit from a clearer understanding of the impact of the decision-making process and actions taken by the NIH during the earliest weeks of the pandemic. To that end, we also recommend that Congress initiate a report from the Government Accountability Office to illuminate the outcomes and learnings of fast governmental programs during COVID-19, such as RADx.
Challenge and Opportunity
The urgency of the COVID-19 pandemic created adaptations not only in how we structure our daily lives but in how we develop therapeutics and fund science. Starting in 2020, the public saw a rapid emergence of nongovernmental programs like Fast Grants, Impetus Grants, and Reproductive Grants to fund both big clinical trials and proof-of-concept scientific studies within timelines that were previously thought to be impossible. Within the government, the NIH launched RADx, a program for the rapid development of coronavirus diagnostics with significantly accelerated approval timelines. Though the sudden onset of the pandemic was unique, we believe that an array of other biomedical crises deserve the same sense of urgency and innovation. It is therefore vital that the new NIH director permanently integrate fast funding programs like RADx into the NIH in order to better respond to these crises and accelerate research progress for the future.
To demonstrate why, we must remember that the coronavirus is far from being an outlier—in the last 20 years, humanity has gone through several major pandemics, notably swine flu, SARS CoV-1, and Ebola. Based on the long-observed history of infectious diseases, the risk of pandemics with an impact similar to that of COVID-19 is about two percent in any year. An extension of naturally occurring pandemics is the ongoing epidemic of opioid use and addiction. The rapidly changing landscape of opioid use—with overdose rates growing rapidly and synthetic opioid formulations becoming more common—makes slow, incremental grantmaking ill-suited for the task. The counterfactual impact of providing some awards via faster funding mechanisms in these cases is self-evident: having tests, trials, and interventions earlier saves lives and saves money, without sacrificing additional resources.
Beyond acute crises, there are strong longer-term public health motivations for achieving faster funding of science. In about 10 years, the United States will have more seniors (people aged 65+) than children. This will place substantial stress on the U.S. healthcare system, especially given that two-thirds of seniors suffer from more than one chronic disease. New disease treatments may help, but it often takes years to translate the results of basic research into approved drugs. The idiosyncrasies of drug discovery and clinical trials make them difficult to accelerate at scale, but we can reliably accelerate drug timelines on the front end by reducing the time researchers spend in writing and reviewing grants—potentially easing the long-term stress on U.S. healthcare.
The existing science funding system developed over time with the best intentions, but for a variety of reasons—partly because the supply of federal dollars has not kept up with demand—administrative requirements have become a major challenge for many researchers. According to surveys, working scientists now spend 44% of their research time on administrative activities and compliance, with roughly half of that time spent on pre-award activities. Over 60% of scientists say administrative burden compromises research productivity, and many fear it discourages students from pursuing science careers. In addition, the wait for funding can be extensive: one of the major NIH grants, R01, takes more than three months to write and around 8–20 months to receive (see FAQ). Even proof-of-concept ideas face onerous review processes and take at least a year to fund. This can bottleneck potentially transformative ideas, as with Katalin Kariko famously struggling to get funding for her breakthrough mRNA vaccine work when it was at its early stages. These issues have been of interest for science policymakers for more than two decades, but with little to show for it.
Though several nongovernmental organizations have attempted to address this need, the model of private citizens continuously fundraising to enable fast science is neither sustainable nor substantial enough compared to the impact of the NIH. We believe that a coordinated governmental effort is needed to revitalize American research productivity and ensure a prompt response to national—and international—health challenges like naturally occurring pandemics and imminent demographic pressure from age-related diseases. The new NIH director has an opportunity to take bold action by making faster funding programs a priority under their leadership and a keystone of their legacy.
The government’s own track record with such programs gives grounds for optimism. In addition to the aforementioned RADx program at NIH, the National Science Foundation (NSF) runs the Early-Concept Grants for Exploratory Research (EAGER) and Rapid Response Research (RAPID) programs, which can have response times in a matter of weeks. Going back further in history, during World War II, the National Defense Research Committee maintained a one-week review process.
Faster grant review processes can be either integrated into existing grant programs or rolled out by institutes in temporary grant initiatives responding to pressing needs, as the RADx program was. For example, when faced with data falsification around the beta amyloid hypothesis, the National Institute of Aging (NIA) could leverage fast grant review infrastructure to quickly fund replication studies for key papers, without waiting for the next funding cycle. In case of threats to human health due to toxins, the National Institute of Environmental Health Sciences (NIEHS) could rapidly fund studies on risk assessment and prevention, giving public evidence-based recommendations with no delay. Finally, empowering the National Institute of Allergy and Infectious Diseases (NIAID) to quickly fund science would prepare us for many yet-to-come pandemics.
Plan of Action
The NIH is a decentralized organization, with institutes and centers (ICs) that each have their own mission and focus areas. While the NIH Office of the Director sets general policies and guidelines for research grants, individual ICs have the authority to create their own grant programs and define their goals and scope. The Center for Scientific Review (CSR) is responsible for the peer review process used to review grants across the NIH and recently published new guidelines to simplify the review criteria. Given this organizational structure, we propose that the NIH Office of the Director, particularly the Office of Extramural Research, assess opportunities for both NIH-wide and institute-specific fast funding mechanisms and direct the CSR, institutes, and centers to produce proposed plans for fast funding mechanisms within one year. The Director’s Office should consider the following approaches.
Approach 1. Develop an expedited peer review process for the existing R21 grant mechanism to bring it more in line with the NIH’s own goals of funding high-reward, rapid-turnaround research.
The R21 program is designed to support high-risk, high-reward, rapid-turnaround, proof-of-concept research. However, it has been historically less popular among applicants compared to the NIH’s traditional research mechanism, the R01. This is in part due to the fact that its application and review process is known to be only slightly less burdensome than the R01, despite providing less than half of the financial and temporal support. Therefore, reforming the application and peer review process for the R21 program to make it a fast grant–style award would both bring it more in line with its own goals and potentially make it more attractive to applicants.
All ICs follow identical yearly cycles for major grant programs like the R21, and the CSR centrally manages the peer review process for these grant applications. Thus, changes to the R21 grant review process must be spearheaded by the NIH director and coordinated in a centralized manner with all parties involved in the review process: the CSR, program directors and managers at the ICs, and the advisory councils at the ICs.
The track record of federal and private fast funding initiatives demonstrates that faster funding timelines can be feasible and successful (see FAQ). Among the key learnings and observations of public efforts that the NIH could implement are:
- Pilot monthly or bimonthly study section and advisory council meetings for R21 grant review. CSR has switched to conducting the majority of its meetings virtually since the COVID-19 pandemic and has found that in-person and virtual meetings are of equal quality. CSR should take advantage of the convenience of virtual meetings by piloting shorter, virtual monthly or bimonthly study section meetings to review R21 grants outside of the three regular meetings held each year. By meeting more frequently but for shorter amounts of time, the individual time commitment for each meeting is reduced, which may incentivize more researchers to participate in study sections and prevent reviewer fatigue from the traditional one- to two-day meetings. To match this change, the advisory councils of ICs that review R21 grant applications should also pilot monthly virtual meetings, timed to occur immediately after the corresponding peer review meetings. Together, these changes could reduce the R21 grant review timeline from a minimum of nine months down to just two or three months.
- Explore new approaches for reviewer participation. One obstacle to faster funding timelines is the recruitment of reviewers without a conflict of interest. Previously, the travel and financial burden of in-person study sections kept the standing body of reviewers small; this makes it difficult to find and gather a quorum of knowledgeable and unconflicted experts. With online study sections, the CSR could engage a larger committee of reviewers at lower cost. This would allow them to identify and address conflicts of interest dynamically and to select a small and varying subset of reviewers to meet each month. Scientists may also be more inclined to participate as potential reviewers, knowing that they may not be called upon for every round of reviews.
- Emphasize the potential value of success over risk. Reviewers should be explicitly instructed not to lower their scores for the Approach criterion (or the new Rigor and Feasibility criterion proposed by CSR) solely due to a lack of extensive prior literature or over differences in the applicant’s past area of expertise. (Reviewer suggestions could still be used to help inform the direction of the proposed work in these cases.) Instead, the Significance and Innovation criteria (or the new Importance of Research criterion) should be weighed much more heavily than other criteria in the overall score. The rationale for these changes is evident: novel areas will naturally have less extensive prior literature, while learnings from one area of research can cross-pollinate innovation in an entirely different area of research. Acceptance of high-risk, high-reward proposals could be further facilitated by piloting the “golden ticket” model, in which reviewers are provided the right to unilaterally fund one application per year that they believe holds the most innovation potential.
- Reduce the length of applications. The length of proposals for both Fast Grants and Impetus Grants did not exceed two pages, which, according to reviewers, was more than enough to make well-reasoned judgment calls. The NIH should reduce the page limit from six to three pages for the R21 grant program. This will reduce the administrative burden and save time for both applicants and peer reviewers.
Pending the success of these changes, the NIH should consider applying similar changes to other major research grant programs.
Approach 2. Direct NIH institutes and centers to independently develop and deploy programs with faster funding timelines using Other Transaction Authority (OTA).
Compared to reforming an existing mechanism, the creation of institute-specific fast funding programs would allow for context-specific implementation and cross-institute comparison. This could be accomplished using OTA—the same authority used by the NIH to implement COVID-19 response programs. Since 2020, all ICs at the NIH have had this authority and may implement programs using OTA with approval from the director of NIH, though many have yet to make use of it.
As discussed previously, the NIA, NIDA, and NIAID would be prime candidates for the roll-out of faster funding. In particular, these new programs could focus on responding to time-sensitive research needs within each institute or center’s area of focus—such as health crises or replication of linchpin findings—that would provide large public benefits. To maintain this focus, these programs could restrict investigator-initiated applications and only issue funding opportunity announcements for areas of pressing need.
To enable faster peer review of applications, ICs should establish (a) new study section(s) within their Scientific Review Branch dedicated to rapid review, similar to how the RADx program had its own dedicated review committees. Reviewers who join these study sections would commit to short meetings on a monthly or bimonthly basis rather than meeting three times a year for one to two days as traditional study sections do. Additionally, as recommended above, these new programs should have a three-page limit on applications to reduce the administrative burden on both applicants and reviewers.
In this framework, we propose that the ICs be encouraged to direct at least one percent of their budget to establish new research programs with faster funding processes. We believe that even one percent of the annual budget is sufficient to launch initial fast grant programs funded through National Institutes. For example, the National Institute of Aging had an operating budget of $4 billion in the 2022 fiscal year. One percent of this budget would constitute $40 million for faster funding initiatives, which would be on the order of initial budgets of Impetus and Fast Grants ($25 million and $50 million accordingly).
NIH ICs should develop success criteria in advance of launching new fast funding programs. If the success criteria are met, they should gradually increase the budget and expand the scope of the program by allowing for investigator-initiated applications, making it a real alternative to R01 grants. A precedent for this type of grant program growth is the Maximizing Investigators’ Research Award (MIRA) (R35) grant program within the National Institute of General Medical Sciences (NIGMS), which set the goal of funding 60% of all R01 equivalent grants through MIRA by 2025. In the spirit of fast grants, we recommend setting a deadline on how long each institute can take to establish a fast grants program to ensure that the process does not extend for too many years.
Additional recommendation. Congress should initiate a Government Accountability Office report to illuminate the outcomes and learnings of governmental fast funding programs during COVID-19, such as RADx.
While a number of published papers cite RADx funding, the program’s overall impact and efficiency haven’t yet been assessed. We believe that the agency’s response during the pandemic isn’t yet well-understood but likely played an important role. Illuminating the learnings of these interventions would greatly benefit future emergency fast funding programs.
Conclusion
The NIH should become a reliable agent for quickly mobilizing funding to address emergencies and accelerating solutions for longer-term pressing issues. As present, no funding mechanisms within NIH or its branch institutes enable them to react to such matters rapidly. However, both public and governmental initiatives show that fast funding programs are not only possible but can also be extremely successful. Given this, we propose the creation of permanent fast grants programs within the NIH and its institutes based on learnings from past initiatives.
The changes proposed here are part of a larger effort from the scientific community to modernize and accelerate research funding across the U.S. government. In the current climate of rapidly advancing technology and increasing global challenges, it is more important than ever for U.S. agencies to stay at the forefront of science and innovation. A fast funding mechanism would enable the NIH to be more agile and responsive to the needs of the scientific community and would greatly benefit the public through the advancement of human health and safety.
The NIH released a number of Notices of Special Interest to allow emergency revision to existing grants (e.g., PA-20-135 and PA-18-591) and a quicker path for commercialization of life-saving COVID technologies (NOT-EB-20-008). Unfortunately, repurposing existing grants reportedly took several months, significantly delaying impactful research.
The current scientific review process in NIH involves multiple stakeholders. There are two stages of review at NIH, with the first stage being conducted by a Scientific Review Group that consists primarily of nonfederal scientists. Typically, Center for Scientific Review committees meet three times a year for one or two days. This way, the initial review starts only four months after the proposal submission. Special Emphasis Panel meetings that are not recurring take even longer due to panel recruitment and scheduling. The Institute and Center National Advisory Councils or Boards are responsible for the second stage of review, which usually happens after revision and appeals, taking the total timeline to approximately a year.
Because of the difficulty of empirically studying drivers of scientific impact, there has been little research evaluating peer review’s effects on scientific quality. A Cochrane systematic review from 2007 found no studies directly assessing review’s effects on scientific quality, and a recent Rand review of the literature in 2018 found a similar lack of empirical evidence. A few more recent studies have found modest associations between NIH peer review scores and research impact, suggesting that peer review may indeed successfully identify innovative projects. However, such a relationship still falls short of demonstrating that the current model of grant review reliably leads to better funding outcomes than alternative models. Additionally, some studies have demonstrated that the current model leads to variable and conservative assessments. Taken together, we think that experimentation with models of peer review that are less burdensome for applicants and reviewers is warranted.
Intuitively, it seems that having longer grant applications and longer review processes ensures that both researchers and reviewers expend great effort to address pitfalls and failure modes before research starts. However, systematic reviews of the literature have found that reducing the length and complexity of applications has minimal effects on funding decisions, suggesting that the quality of resulting science is unlikely to be affected.
Historical examples have also suggested that the quality of an endeavor is largely uncorrelated from its planning times. It took Moderna 45 days from COVID-19 genome publication to submit the mRNA-1273 vaccine to the NIH for use in its Phase 1 clinical study. Such examples exist within government too: during World War II, National Defense Research Committee set a record by reviewing and authorizing grants within one week, which led to DUKW, Project Pigeon, Proximity fuze, and Radar.
Recent fast grant initiatives have produced high-quality outcomes. With its short applications and next-day response times, Fast Grants enabled:
- detection of new concerning COVID-19 variants before other sources of funding became available.
- work that showed saliva-based COVID-19 tests can work just as well as those using nasopharyngeal swabs.
- drug-repurposing clinical trials, one of which identified a generic drug reducing hospitalization from COVID-19 by ~40%.
- Research into “Long COVID,” which is now being followed up with a clinical trial on the ability of COVID-19 vaccines to improve symptoms.
Impetus Grants focused on projects with longer timelines but led to a number of important preprints in less than a year from the moment person applied:
- Aging Fly Cell Atlas
- Modular, programmable RNA sensing using ADAR editing in living cells
- Mechanisms of natural rejuvenation in a test tube
- Optogenetic rejuvenation of mitochondrial membrane potential to extend C. elegans lifespan
- Evidence that conserved essential genes are enriched for pro-longevity factors
- Trials on neuroprotective effects of Canagliflozin
With the heavy toll that resource-intensive approaches to peer review take on the speed and innovative potential of science—and the early signs that fast grants lead to important and high-quality work—we feel that the evidentiary burden should be placed on current onerous methods rather than the proposed streamlined approaches. Without strong reason to believe that the status quo produces vastly improved science, we feel there is no reason to add years of grant writing and wait times to the process.
The adoption of faster funding mechanisms would indeed be valuable across a range of federal funding agencies. Here, we focus on the NIH because its budget for extramural research (over $30 billion per year) represents the single largest source of science funding in the United States. Additionally, the NIH’s umbrella of health and medical science includes many domains that would be well-served by faster research timelines for proof-of-concept studies—including pandemics, aging, opioid addiction, mental health, cancer, etc.
Building Momentum for Equity in Medical Devices
Just over a year ago, I found myself pausing during a research lab meeting. “Why were all the subjects in our studies of wearable devices white? And what were the consequences of exclusion?”
This question stuck with me long after the meeting. Digging into the evidence, I was alarmed to find paper after paper signaling embedded biases in key medical technologies.
One device stuck out amongst the rest – the pulse oximeter. Because of its crucial role in diagnosing COVID-19, it had caught the attention of a diverse group of stakeholders: clinicians looking to understand the impacts on patient care, engineers working to build more equitable devices, social scientists tracing the history of device and examining colorism in pulse oximetry, policymakers seeking solutions for their constituents, and the FDA, which was examining racial bias in medical technologies for the first time. But what I found as I scoped out this policy area is that these stakeholders weren’t talking to one another, at the expense of coordinated progress towards equity in pulse oximetry.
With all eyes directed towards the FDA’s Advisory Committee meeting on November 1st, 2022, FAS convened a half-day session of stakeholders on November 2nd to chart a research and policy agenda for near-term mitigation of inequities in pulse oximetry and other medical technologies. Eight experts from medicine, engineering, sociology, and anthropology shared insights with an audience of 60 participants from academia, the private sector, and federal government. Collectively, we developed several key insights for future progress on this issue and outlined a path forward for achieving equity now. You can access the full readout here. We’ll dive into the key highlights below:
Key Insights
Through discussions with experts during the forum, three key themes rose to the surface:
- Racial bias in pulse oximetry cannot be fixed by focusing on “race” alone. Existing evidence suggests reducing bias in pulse oximetry requires replacing devices with less-biased ones. This will take time as new devices are developed and will be a significant cost.
- Better calibration for skin tone is vital, but measurement is complicated. The crux of the problem is a comprehensive standard for quantifying the full range of skin pigmentation. This is vital to understanding how pulse oximeter accuracy varies by melanin content.
- Proactively identifying and addressing bias in medical devices will require system-wide efforts. Identification of bias in medical devices has been piecemeal rather than the outcome of proactive, deliberative efforts. Further efforts to address bias in medical devices should engage diverse stakeholders to establish best practices for ensuring equity in medical devices.
Resolving the problem of bias in pulse oximeter devices will likely take several years. But in the meantime, this issue will continue negatively impacting patients. Our participants urged that we need to think about actions that can be initiated this next year that will advance more equitable care with existing pulse oximeters.

Motivating Action for Equity Now
While a daunting problem, a collaborative, multi-stakeholder effort can bring us closer to solutions. We can work together to advance equity in standards of care by:
- Gathering evidence on existing pulse oximeter devices and their use in care [ASAP, start early 2023]. More evidence is required to identify the best approaches to equitable care with existing devices. This evidence gathering process should be initiated over the next year to inform clinicians on
- Establishing consensus to advance the standard of care [start early 2024]. After growing the body of evidence, there will be a need to convene around key conclusions derived from the evidence. Evidence synthesis will need to be generated and care societies will need to make decisions on how clinicians should use pulse oximeters in their care practice.
- Taking action to ensure equitable care nationwide [2024 onwards]. Once the care standards are changed, there is a need for system-wide efforts to communicate these to clinicians nationwide, inform procurement across federal hospitals, and re-evaluate insurance reimbursement standards.
Looking Ahead
This won’t be easy, but it’s 30 years overdue. We believe correcting the bias will pioneer a model that can be readily applied to combatting biases across the medical device ecosystem, something already underway in the United Kingdom with their Equity in Medical Devices Independent Review. Through a systematic approach, stakeholders can work to close racial disparities in the near-term and advance health equity.
Saving 3.1 Million Lives a Year with a President’s Emergency Plan to Combat Acute Childhood Malnutrition
Summary
Like HIV/AIDS, acute childhood malnutrition is deadly but easily treatable when the right approach is taken. Building on the success of PEPFAR, the Biden-Harris Administration should launch a global cross-agency effort to better fund, coordinate, research, and implement malnutrition prevention and treatment programs to save millions of children’s lives annually and eventually eliminate severe acute malnutrition.
Children with untreated severe acute malnutrition are 9 to 11 times more likely to die than their peers and suffer from permanent setbacks to their neurodevelopment, immune system, and future earnings potential if they survive. Effective programs can treat children for around $60 per child with greater than 90 percent recovery rates. However, globally, only about 25–30 percent of children with moderate and severe acute malnutrition have access to treatment. Every year, 3.1 million children die due to malnutrition-related causes, and 45% of all deaths of children under five are related to malnutrition, making it the leading cause of under-five deaths.
In 2003, a similar predicament existed: the HIV/AIDS epidemic was causing millions of deaths in sub-Saharan Africa and around the world, despite the existence of highly effective treatment and prevention methods. In response, the Bush Administration created the President’s Emergency Plan for AIDS Relief (PEPFAR). PEPFAR has proven a major global health success, saving an estimated 30 million lives since 2003 through over $100 billion in funding.
The Biden-Harris Administration should establish a President’s Emergency Plan for Acute Childhood Malnutrition (PEPFAM) in the Office of Global Food Security at the State Department to clearly elevate the problem of acute childhood malnutrition, leverage new and existing food security and health programs to serve U.S. national security and humanitarian interests, and save the lives of up to 3.1 million children around the world, every year. PEPFAM could serve as a catalytic initiative to harmonize the fight against malnutrition and direct currently fragmented resources toward greater impact.
Challenge and Opportunity
United Nations Sustainable Development Goal (SDG) 2.2 outlines goals for reducing acute malnutrition, ambitiously targeting global rates of 5 percent by 2025 and 3 percent (a “virtual elimination”) by 2030. Due to climate change, the COVID-19 pandemic, and conflicts like the war in Ukraine, global rates of malnutrition remain at 8 percent and are forecast to become worse, not better. Globally, 45.4 million children suffer from acute malnutrition, 13.6 million of whom are severely acutely malnourished (SAM). If current trends persist until 2030, an estimated 109 million children will suffer from permanent cognitive or physiological stunting, despite the existence of highly effective and relatively cheap treatment.
Providing life-saving treatment around the world serves a core American value of humanitarianism and helps meet commitments to the SDGs. The United States Agency for International Development (USAID) recently announced a commitment to purchase ready-to-use therapeutic food (RUTF), a life-saving food, on the sidelines of the UN General Assembly, demonstrating a prioritization of global food security. Food security is also a priority for the Biden Administration’s approach to national security. The newly released National Security Strategy dedicates an entire section to food insecurity, highlighting the urgency of the problem and calling on the United States and its global partners to work to address acute needs and tackle the extraordinary humanitarian burden posed by malnutrition. The Office of Global Food Security at the U.S. Department of State also prioritizes food security as an issue of national security, leading and coordinating diplomatic engagement in bilateral, multilateral, and regional contexts. At a time when the United States is competing for its vision of a free, open, and prosperous world, addressing childhood malnutrition could serve as a catalyst to achieve the vision articulated in the National Security Strategy and at the State Department.
“People all over the world are struggling to cope with the effects of shared challenges that cross borders—whether it is climate change, food insecurity, communicable diseases, terrorism, energy shortages, or inflation. These shared challenges are not marginal issues that are secondary to geopolitics. They are at the very core of national and international security and must be treated as such.”
U.S. 2022 National Security Strategy
Tested, scalable, and low-cost solutions exist to treat children with acute malnutrition, yet the platform and urgency to deliver interventions at scale does not. Solutions such as community management of acute malnutrition (CMAM), the gold standard approach to malnutrition treatment, and other intentional strategies like biofortification could dramatically lower the burden of global childhood malnutrition. Despite the 3.1 million preventable deaths that occur annually related to childhood malnutrition and the clear threat that food insecurity poses to U.S. national security, we lack an urgent platform to bring these low-cost solutions to bear.
While U.S. government assistance to combat food insecurity and malnutrition is a priority, funding and coordination are not centralized. The U.S. has committed over $10 billion to address global food insecurity, allocating dollars to USAID, Feed the Future, the U.S. Department of Agriculture (USDA), and others. Through the recently signed Global Malnutrition Prevention and Treatment Act of 2021, Congress took a step forward by authorizing USAID to have greater authority in targeting nutrition aid to areas of greatest need and greater flexibility to coordinate activities across the agency and its partners. In accordance with the agency’s Global Nutrition Coordination Plan, Congress also established the Nutrition Leadership Council, chaired by the Bureau for Resilience and Food Security to coordinate and integrate activities solely within USAID. Multilateral and private sector partners also dedicate resources to food security: the Gates Foundation committed $922 million toward global nutrition and food systems, and UNICEF created a Nutrition Match Fund to incentivize funding to combat severe acute malnutrition. These lines of effort are each individually important, but could be more impactful if aligned. A President’s Emergency Plan for malnutrition could harmonize these separate funding streams and authorities and mobilize multilateral and private sector partners to prevent and treat malnutrition and food insecurity.
Drawing on the strengths of the PEPFAR model to combat HIV/AIDS at scale while driving down costs for treatment, PEPFAM could revolutionize how resources are spent while scaling sustainable and cost-effective solutions to childhood malnutrition, saving millions of lives every year. Under this model, significantly more—and, optimally, all—children suffering from acute malnutrition would have access to treatment. This would make dramatic progress toward global food security and U.S. national security priorities.
Plan of Action
President Biden should declare a global childhood malnutrition emergency and announce the creation of the President’s Emergency Plan for Acute Childhood Malnutrition. Using PEPFAR as a model, PEPFAM could catalyze cost-effective solutions to save millions of lives every year. When President Bush mobilized support for PEPFAR in his 2003 State of the Union, he declared, “We must remember our calling, as a blessed country, is to make the world better,” and called for interagency support for an “Emergency Plan” for HIV/AIDS relief and Congressional support to commit $15 billion over the next five years to launch PEPFAR.
President Biden should follow a similar path and announce PEPFAM in a similarly high-profile speech—the 2023 State of the Union address, for example—to elevate the problem of acute childhood malnutrition to the American people and the U.S. government and offer a clear call to action through an executive order directing an interagency task force to develop a 24-month strategic plan within 180 days. The initial stages of PEPFAM and corresponding executive branch activities can be guided by the following recommendations.
Recommendation 1. Name a White House PEPFAM czar and task the Office of Global Food Security at the State Department to coordinate cross-agency support, intended personnel, agencies, and roles involved.
A Senior Advisor on the White House’s National Security Team at the Office of Science and Technology Policy would serve as the White House czar for PEPFAM and would (1) steer and lead the initiative, (2) organize an interagency task force, and (3) coordinate PEPFAM’s strategic focus by engaging multiple federal agencies, including:
- USAID’s Bureau of Resilience and Food Security
- State Department’s Office of Global Food Security
- State Department’s Office of the Global AIDS Coordinator and Health Diplomacy (OGAC)
- Department of Health and Human Service’s Office of Global Affairs
- Department of Agriculture’s Foreign Agricultural Service
- The White House National Security Council (and/or other relevant offices)
The Office of the Global AIDS Coordinator and Health Diplomacy at the State Department (OGAC) manages the high-level execution of PEPFAR by dictating strategic direction and coordinating agencies. The PEPFAM executive order will set up a similar infrastructure at the Office of Global Food Security at the State Department to:
- Coordinate activities and funding across the U.S. government, the private sector, and multilateral organizations
- Approve all activities related to PEPFAM
- Oversee accountability and monitoring and evaluation
- Provide strategic direction for the program
USAID is also well positioned to play a leading role given its current support of global food and nutrition programming. Several of USAID’s portfolios are central to PEPFAM’s aims, including Agriculture and Food Security, Nutrition, Global Health, Water and Sanitation, and Humanitarian Assistance. The offices that support these portfolios should provide technical expertise in the realm of food and nutrition, existing connections to good program implementers in various country contexts, monitoring and evaluation capacity to track implementer’s progress toward goals, and strategic direction.
The Office of Global Food Security and the PEPFAM czar should delegate authority for the program across government agencies, private partners (e.g., Gates Foundation), and multilateral organizations (e.g., World Food Programme). The Office would coordinate interagency action to support PEPFAM’s implementation and evaluation as well as identify agencies that are best placed to lead each component of the effort.
Recommendation 2. Present initial, strategic action plan to build and sustain PEPFAM.
The PEPFAM interagency task force, described above, should develop a strategic plan targeting an initial set of actions to align with existing global food security and childhood malnutrition priorities and identify opportunities to redirect existing resources toward scalable, high-impact solutions like CMAM. USAID already invests millions of dollars each year in initiatives like Feed the Future that support global food security while overseeing cross-agency implementation and harmonization of the Global Food Security Strategy. These efforts and funding should be rolled under the umbrella of PEPFAM to better align treatment and prevention interventions, strategically coordinate resources across the government, and improve a focus on impact.
Recommendation 3. Announce discrete, evidence-driven goals for PEPFAM.
These goals include:
- Catalyze global funding and direct resources toward effective, low-cost solutions to address acute childhood malnutrition.
- Provide sustained access to treatment for children suffering from acute malnutrition, both moderate and severe, even in geographic areas that are not designated as crises or emergency situations.
- Scale proven, cost-effective prevention interventions to reduce the burden of childhood malnutrition and invest in research and evaluation to identify new prevention mechanisms.
- Coordinate and conduct targeted humanitarian efforts to triage and respond rapidly to emergent situations of famine/starvation.
- Invest in research, innovation, and monitoring and evaluation to ensure that U.S. government and global funds are put toward the most cost-effective (e.g., cheap and effective) interventions to maximize the impact of existing and additional funds.
Recommendation 4. Establish a coordination framework between PEPFAM, multilateral agencies, and private sector partners to mobilize and harmonize resources.
The Office of Global Food Security and USAID should build on current momentum to bring multilateral and private partners behind PEPFAM. USAID has recently announced a series of partnerships with large philanthropic organizations like the Gates Foundation, Aliko Dangote Foundation, and Eleanor Crook Foundation (to name a few), as well as other countries and multilateral organizations at UNGA. Much like with PEPFAR, PEPFAM could rely on the support of external partners as well as federal funds to maximize the impact of the program.
Recommendation 5. Create an international council to set technical standards so that money goes to the most effective programs possible.
The Office of Global Food Security, USAID, and PEPFAM should spearhead the development of an international technical council (that could be housed under the UN, the World Health Organization, or independently) to set standards for malnutrition prevention and treatment programming. Malnutrition treatment is already cost-effective, but it could be made even cheaper and more effective through innovation. Even when promising new interventions are identified, the process of disseminating and scaling of existing, proven best practices innovations doesn’t function optimally.
Treatment guidelines issued by the WHO and national governments are slow to be updated, meaning that highly effective interventions can take years to be adopted and, even then, are adopted in a piecemeal fashion. Other implementers may be too wedded to their operational practices to consider making a change unless standards are updated or innovations from other implementers are actively socialized.
An international technical council would disseminate and scale best practices discovered in the processes of implementation and research. If funders like the U.S. government commit to only funding organizations that promptly adopt these standards, they can maximize the impact of existing funding by ensuring that every dollar goes toward the most cost-effective ways of saving lives. This body could ideally speed the sharing and implementation of practices that could allow more children to be treated effectively, at lower costs.
Recommendation 6. Direct existing child malnutrition assistance through PEPFAM to ensure coordinated impact and seek permanent funding from Congress for PEPFAM.
The executive order will create the momentum to establish PEPFAM, but legislative authorization is required to make it sustainable. The strategic plan should lay out efforts to build Congressional support for funding legislation.
Congress will play a key role in PEPFAM implementation by appropriating funds. Under PEPFAR, Congress appropriates money directly to OGAC at the Department of State, which disburses it to other agencies. In 2003, Congress supported President Bush’s request for $15 billion in PEPFAR funding by passing the Leadership Act that authorized yearly contributions to the Global Fund from 2004 to 2008. Congress has subsequently reauthorized the program through FY2023. Each year, the OGAC presents a request of funding needed for recipient countries and programs to the President, who then forwards the request to Congress for reauthorization. The PEPFAM process should mirror this structure.
At the UNGA in 2022, President Biden announced over $2.9 billion in new assistance to address global food insecurity, building on the $6.9 billion in U.S. government assistance already committed in 2021. Last year, President Biden also announced a $10 billion, multiyear investment to promote food systems transformation, including a $5 billion commitment to Feed the Future specifically. Instead of fractured funding to different initiatives, these funds should be harmonized under PEPFAM, with dollars allocated to the PEPFAM task force to create a centralized two-year strategy to combat malnutrition.
Conclusion
This program would have a series of positive effects. First, and most obviously, PEPFAM would save up to 3.1 million lives every year and bring together resources and goals around food security that are currently fractured across the federal government, increasing the effectiveness of U.S. aid dollars globally. Second, PEPFAM, like PEPFAR, would make existing interventions more effective by unlocking cost savings and innovation at scale. Third, at a time when the United States is competing for its vision of a free, open, and prosperous world, PEPFAM could play a key role in achieving the mission of the National Security Strategy.
Over time, more comprehensive treatment coverage and prevention efforts could also lead to the elimination of severe acute malnutrition by preventing cases and catching those that approach moderate acute malnutrition or have already fallen into it. PEPFAM would save an estimated 27.9 million lives over the same time scale as PEPFAR. Millions of children die every year while a cheap and effective solution exists. PEPFAM could change that.
From 2003 to present day, PEPFAR has spent billions of dollars and saved millions of lives. This table compares the estimated costs and outcomes of PEPFAR with PEPFAM. Because malnutrition treatment is cheaper than HIV/AIDS treatment and there is a higher caseload, there is a high-leverage opportunity to save lives.
| PEPFAR (HIV/AIDS) | PEPFAM (Childhood Malnutrition) | |
| Average Cost of Treatment per Person | $367,134 | $60 |
| Number of Cases | 38.4 million | 45.4 million |
| Program Cost (estimated yearly) | $5.7 billion (USD) | $4 billion (USD) |
| Lives Saved (estimated yearly) | 1.6 million | 1.5 million |
Costs for PEPFAM are difficult to project precisely, because the program is likely to become more cost-effective over time as efforts to prevent cases start to work and research and development result in cheaper and more effective treatment. The projections above operate under the most pessimistic assumptions that no improvements to cost or effectiveness are made over time. This graph illustrates a similar the expansion of PEPFAR services, even under flat budgets thanks to this same self-improvement over time.

PEPFAM is similar: more comprehensive treatment coverage and prevention efforts could lead to the elimination of severe acute malnutrition by preventing cases and catching those that approach moderate acute malnutrition or have already fallen into it. That means that the program should become cheaper over time, as more cases are identified earlier when they are cheaper to treat, and more cases are prevented, both by prevention programs and general economic development. Research and innovation can similarly cut down on the costs and improve the effectiveness of malnutrition treatment and prevention over time.
The lack of attention to childhood malnutrition in non-emergency/non-crisis zones results in millions of preventable deaths each year. Declaring an emergency would put pressure on other organizations, media outlets, and NGOs to devote more resources to food security. The international community is keen to respond to food crises in emergency contexts, especially among children. USAID and the UN recently committed millions of dollars for the procurement of ready-to-use therapeutic food (RUTF) to combat emergency risks like the war in Ukraine and conflicts in places like Ethiopia. But the unfortunate truth is that even outside of newsworthy emergencies, acute malnutrition remains a daily emergency in many places around the world. Malnutrition rates are just as high in states and countries that neighbor emergency zones as in the crisis-hit places themselves, partially as a result of movement of internally displaced people. While funding acute malnutrition in relatively mundane circumstances (e.g., poverty-stricken states in Nigeria) may make less headlines than emergency food aid, it’s equally needed.
Currently, only 1 percent of U.S. global health spending is put toward nutrition. Only 25–30 percent of children globally have access to treatment as a result of underfunded programs and a subsequent lack of resources and geographic coverage.
Treatment is only effective if implemented well. Right now, funding goes to a range of programs that fail to meet Sphere Standards of 75 percent recovery rates. Large-scale funders like UNICEF have internal commitments to spend a certain amount of their budgets on ready-to-use therapeutic food (RUTF) a year, which means that their hands are tied when working in contexts with poor implementing partners (e.g., corrupt governments). At the same time, NGOs like Alliance for International Medical Action and Médecins Sans Frontières achieve recovery rates of more than 95 percent. More investment in quality implementation capacity is needed; otherwise, scarce existing resources will continue to be wasted.
There’s a growing movement to implement interventions that catch children on the border of malnutrition or improve conditions that lead to malnutrition in the first place (e.g., infant and young child feeding circles, exclusive breastfeeding counseling). These programs are exciting, but the evidence base for impact at this point is minimal. It’s much cheaper to catch a child before they fall into malnutrition than it is to treat them, not to mention the health benefits to the child from averting the disease. More work needs to be done to test and validate the most cost-effective prevention methods to ensure that only those that actually generate impact are scaled.
Childhood malnutrition sits at the intersection of public health and nutrition/agricultural programming. Current efforts are spread across the U.S. government and multilateral partners with little coordination toward desired outcomes. Funding that hypothetically targets childhood malnutrition can come from a variety of players in the U.S. government, ranging from Department of Defense to USAID to the Department of Agriculture. While some coordination through programs like Feed the Future exist at USAID, these programs are not yet results- or outcome-based. Coordination should involve measuring the impact of collective aid across agencies on an outcome like recovery rates or the number of children suffering from malnutrition in a given geographic area.