Translating Vision into Action: FAS Commentary on the NSCEB Final Report and the Future of U.S. Biotechnology
Advancing the U.S. leadership in emerging biotechnology is a strategic imperative, one that will shape regional development within the U.S., economic competitiveness abroad, and our national security for decades to come. In the past few years, the contribution of biotechnology to the U.S. economy (referred to as the bioeconomy) has grown significantly, contributing over $210 billion to GDP and creating more than 640,000 domestic jobs, cementing its role as a major and expanding economic force. The impact of biotechnologies and biomanufacturing can be seen across diverse sectors and geographies, with applications spanning agriculture, energy, industrial manufacturing, and health. As biotechnology continues to drive innovation, it is emerging as a core engine of the next industrial revolution.
To maximize the strategic potential of emerging biotechnology, Congress established the bipartisan National Security Commission on Emerging Biotechnology (NSCEB) through the FY22 National Defense Authorization Act. The commission was tasked with conducting a comprehensive review of how advancements in biotechnology and related technologies will shape the current and future missions of the Department of Defense (DOD), and developing actionable policy recommendations to support the adoption and advancement of biotechnology within DOD and across the federal government. This effort culminated in a report, “Charting the Future of Biotechnology”, delivered to Congress in April 2025. The final report outlines 49 recommendations aimed at accelerating biotechnology innovation and scaling the U.S. biomanufacturing base, reinforcing the bioeconomy as a strategic pillar of national security and economic competitiveness.
In addition to developing a series of recommendations to promote and grow the U.S. bioeconomy, the Commission has also been tasked with facilitating the implementation and adoption of these policy recommendations by Congress and relevant federal agencies. To date, several pieces of legislation have been introduced in both the 118th and 119th Congress that incorporate recommendations from NSCEB’s interim and final reports (Table 1). This Legislation Tracker will be updated as this legislation moves through the process and as new bills are introduced.
The NSCEB report represents a critical policy opportunity for the U.S. bioeconomy. It proposes an injection of $15 billion to support sustained growth in biotechnology innovation and biomanufacturing through strategic investment and improved coordination. This level of investment is significant and would signal congressional support for the bioeconomy that goes beyond that seen in CHIPS and Science Act and the Inflation Reduction Act of 2022. This much needed infusion of federal investment offers a timely opportunity to build on existing momentum and unlock the next phase of U.S. leadership in the bioeconomy.
The recommendations in the report should be seen as opportunities for engagement with the Commission and with Congress for further refinement of these policy ideas. As the Commission begins its work on implementation, they have called on stakeholders across the bioeconomy to help refine and strengthen its proposals. Responding to this need, the Federation of American Scientists (FAS) has identified priority areas requiring greater clarity and has issued an open call for supplemental recommendations and policy proposals through the Day One Open Call process.
Overall, FAS supports the Commission’s final report and we applaud the Commission’s efforts to elevate the national conversation around emerging biotechnologies. The report provides a necessary foundation for long-term federal strategy and investment in biotechnology and biomanufacturing. At the same time, there remain clear opportunities to strengthen the recommendations through greater specificity and deeper stakeholder engagement. Two overarching decisions by the Commission deserve some additional scrutiny. First, the report’s adversarial framing towards China, while grounded in strategic reality, risks overlooking opportunities for targeted collaboration that could yield global benefits, particularly in areas where scientific progress depends on multinational cooperation. Second, the final report gives limited attention to the agricultural sector, despite its clear relevance to national security and the DOD’s growing interest in agricultural biotechnology. The “Additional Considerations” section does include a constructive call to modernize the USDA’s BioPreferred Program and update federal classification systems, recommendations that echo those issued by FAS. A more comprehensive approach toward this sector is needed.
The following sections summarize the report’s key pillars and provide analysis, highlighting core recommendations and identifying opportunities where additional detail and stakeholder input, through the Day One Open Call, will be essential for translating the report’s vision into actionable, high-impact policy. Additionally, the Supplementary Recommendations Table for the NSCEB Final Report (Table 2) lists each of the recommendations from the pillars and cross references related proposals from prior FAS work, subject matter experts, and Day One Memos submitted by external stakeholders.
Table 2: Supplementary Recommendations Table for the NSCEB Final Report
Pillar 1. Prioritize Biotechnology at the National Level
Pillar 1 of the report emphasizes the need to prioritize biotechnology at the national level. The recommendations within this pillar are essential for the development of a cohesive national strategy, and we encourage Congress to consider incorporating terminology and drawing on previous policy related to the bioeconomy to ensure that previous progress related to emerging biotechnologies is not lost.
A central recommendation within this Pillar is the establishment of the National Biotechnology Coordination Office (NBCO), which would reduce fragmentation and elevate biotechnology as a national priority. To succeed, the NBCO must address challenges faced by past coordination bodies and be empowered by the administration to drive cross-agency strategy despite differing institutional perspectives. While the presidential appointment of the director could lend authority, it also risks politicization and strategic shifts that may destabilize the sector. Success will depend on clarity of roles, coordination across functions, and strong institutional support for implementation.
FAS provided several additional recommendations and insights on these topics (see Table 2) to make them more nuanced and actionable by Congress, including:
- Coordinating the U.S. Government Approach to the Bioeconomy by Sarah Carter
- A National Bioeconomy Manufacturing and Innovation Initiative by Alexander Titus
Pillar 2. Mobilize the Private Sector to get U.S. Products to Scale
Pillar 2 of the final report focuses on mobilizing the private sector to strengthen biotechnology products by addressing key challenges to the sector, including regulatory reform, financing obstacles, and infrastructure and data needs. While the report correctly identifies long standing regulatory bottlenecks for products of biotechnology under the Coordinated Framework, including unclear oversight and interagency conflicts, it also acknowledges statutory complexities that make reform difficult. Empowering the Office of Management and Budget’s Office of Information and Regulatory Affairs (OIRA) to mediate these disputes is a promising approach, but would require statutory reinforcement. Similarly, proposals to modernize regulatory capacity, such as agency fellowships and regulatory science programs, highlight a critical need for technical expertise within government, though questions remain about institutional placement and long-term sustainability.
On financing and infrastructure, the report points to real gaps in early-stage capital and scale-up capacity, particularly for bridging the “Valley of Death” for biotechnology manufacturing. Concepts like advance market commitments and a new investment fund have potential, but their impact will depend heavily on design, risk management, and alignment with existing capital pipelines. The infrastructure recommendations are strong, but coordination challenges, particularly among national labs, regional hubs, and entities like BioMADE, must be addressed to avoid duplication or underutilization and approaches to securing bioeconomy infrastructure and data are underdeveloped. It will be critical to better define what constitutes critical biotechnology infrastructure and how it should be protected.
FAS provided significant expertise on these topics (see Table 2), such as:
- Coordinating the U.S. Government Approach to the Bioeconomy by Sarah Carter
- Regulations for the Bioeconomy by Sarah Carter
- A National Frontier Tech Public-Private Partnership to Spur Economic Growth by Katie Rae, Orin Hoffman & Michael Kearney
- De-Risking the U.S. Bioeconomy by Establishing Financial Mechanisms to Drive Growth and Innovation by Nazish Jeffery & Zak Weston
- Advancing the U.S. Bioindustrial Production Sector by Michael Fisher
- Closing Critical Gaps from Lab to Market by Phil Weilerstein, Shaheen Mamawala, and Heath Naquin
- Strengthening the U.S. Biomanufacturing Sector Through Standardization by Chris Stowers
- Summary Report – December 7, 2022, Bioeconomy Policy Workshop: Financial and Economic Tools
- Project BOoST: A Biomanufacturing Test Facility Network for Bioprocess Optimization, Scaling, and Training by Ed Charles & Chris Fracchia
One of the NSCEB’s recommendations in particular would benefit from additional input from external subject matter experts to make it more concrete and actionable for Congress:
- Recommendation 2.2d: Congress should improve the effectiveness and reach of the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs to support early-stage innovation. Specifically, stakeholder input on:
- Which areas of biotechnology or sectors within the bioeconomy would most benefit from SBIR and STTR investment?
- How can these programs better support not only early- but also late-stage innovation?
If you have specific policy suggestions related to this topic, we encourage you to submit your ideas through the Day One Open Call page at FAS.
Pillar 3. Maximize the Benefits of Biotechnology for Defense
Pillar 3 of the final report focuses on maximizing the benefits of biotechnology for national defense, with an emphasis on the intelligence community and the Department of Defense (DOD). This pillar includes recommendations related to BioMADE oversight as well as internal workforce education on biotechnology. While oversight of BioMADE is important, it is unclear why additional oversight from Congress is needed for this manufacturing institute above and beyond that provided by its federal sponsor. Additionally, it will be essential for DOD to establish a mechanism for regularly updating workforce education in biotechnology, given the sector’s rapid and continuous evolution.
More broadly, this Pillar appropriately reframes biotechnology as a strategic capability, beyond its traditional role in R&D. This shift in perspective is timely, but realizing the potential of these technologies will require significant institutional change. Ethical use frameworks, particularly around dual-use risks (warfighter enhancement, surveillance, and environmental impact) must be developed through a transparent process that extends beyond DOD to include external stakeholders and independent organizations. In addition, proposed investment and export controls aimed at limiting adversarial advantage must be carefully scoped and implemented. The Department of Commerce (DOC) has published multiple Requests for Information (2018, 2020) to understand and delineate “high-risk” biotech products. Yet, DOC has not added new biotech products to the export list, which highlights the complexity of this task, and underscores the need for precision to avoid stifling beneficial collaboration or disrupting global supply chains.
FAS provided several additional recommendations and insights on these topics (see Table 2) to make them more nuanced and actionable by Congress, including:
- CLimate Improvements through Modern Biotechnology (CLIMB) — A National Center for Bioengineering Solutions to Climate Change and Environmental Challenges by Jennifer Panlilio & Hanny Rivera
- A Foundational Technology Development and Deployment Office to Create Jobs by Katie Rae, Michael Kearney, and Orin Hoffman
Pillar 4. Out-Innovate our Strategic Competitors
Pillar 4 of the NSCEB’s report offers recommendations for strengthening the biotechnology sector to out-innovate global competitors. It focuses on building robust data ecosystems, enhancing biosecurity, and expanding bio R&D infrastructure within the U.S. A central theme is the creation of a modern biological data ecosystem, which would provide the foundational infrastructure necessary to accelerate innovation. While a biological data ecosystem and associated standards are timely, several technical and governance challenges must be addressed, like harmonizing legacy systems, defining AI-readiness, and coordinating cloud lab integration. These complexities present an opportunity for stakeholder engagement and thoughtful design.
Within the Pillar, proposals that call for expanding National Lab capabilities and funding interdisciplinary biotechnology research are directionally strong, but success will depend on interagency coordination and alignment with industry needs. Finally, the report calls for stronger governance of biosafety and biosecurity, though its assertion that past efforts have “failed” could benefit from more nuanced analysis.
While FAS provided expertise on these topics (see Table 2), such as:
- A National Bioeconomy Manufacturing and Innovation Initiative by Alexander Titus
- Kickstarting Collaborative, AI-Ready Datasets in the Life Sciences with Government-funded Projects by Erika DeBenedictis, Ben Andrew, and Pete Kelly
- Creating a National Exposome Project by Gurdane Bhutani, Gary Miller, and Sandeep Patel
- Accelerating Biomanufacturing and Producing Cost-Effective Amino Acids through a Grand Challenge by Allison Berke
- Accelerating Bioindustry Through Research, Innovation, and Translation by Jon Roberts
A few of the report’s recommendations would benefit from additional stakeholder input to enhance clarity and ensure they are actionable for Congress:
- Recommendation 4.1c: Congress should authorize and fund the Department of Interior to create a Sequencing Public Lands Initiative to collect new data from U.S. public lands that researchers can use to drive innovation and Recommendation 4.1d: Congress should authorize the National Science Foundation to establish a network of “cloud labs,” giving researchers state-of-the-art tools to make data generation easier. Specifically, stakeholder input on:
- What type of data should be generated and what types of data generation or collection should be prioritized?
- How can we best draw on or expand existing cloud lab capabilities?
- Recommendation 4.3b: Congress should initiate a grand research challenge focused on making biotechnology predictably engineerable. Specifically, stakeholder input on:
- What specific grand challenge should Congress pursue and how should it be implemented?
- How should the U.S. government engage the scientific community (and others) in establishing and pursuing grand challenges for biotechnology?
- Recommendation 4.4a: Congress must direct the executive branch to advance safe, secure, and responsible biotechnology research and innovation. Specifically, stakeholder input on:
- The report calls for establishment of a body within the U.S. government for this purpose. What should this look like and how would it operate?
If you have specific policy suggestions related to these topics, we encourage you to submit your ideas through the Day One Open Call at FAS.
Pillar 5. Build the Biotechnology Workforce of the Future
Pillar 5 of the final report looks to the future by offering recommendations to secure and build the biotechnology workforce needed in the future. It addresses both the modernization of the federal biotech workforce and the development of the broader U.S. biotech workforce. Modernizing the federal workforce requires more than training programs. It requires coordination across HR systems, consistent standards, and better integration of biotechnology experts into national security and diplomacy. Proposals to expand Congressional science capacity are long overdue and necessary to equip lawmakers to address rapidly evolving biotechnology issues. On the national level, scaling the biomanufacturing workforce will depend on aligning credentials with industry needs and securing input from labor, academia, and employers. Expanding biotechnology education is promising, but successful implementation will require investment in teacher training and curriculum development.
While FAS contributed several recommendations to support this critical capacity-building effort (see Table 2), such as:
- Gathering Industry Perspectives on how the U.S. Government can Support the Bioeconomy by Sarah Carter
- Meeting Biology’s “Sputnik Moment”: A Plan to Position the United States as a World Leader in the Bioeconomy by Natalie Kuldell
One of the NSCEB’s recommendation would benefit from additional stakeholder input to enhance its clarity and make it more actionable for Congress:
- Recommendation 5.2a: Congress must maximize the impact of domestic biomanufacturing workforce training programs. Specifically, stakeholder input on:
- How should the government approach creating competency models for biomanufacturing training and microcredentialing?
- Which specific areas are best suited for microcredentialing efforts?
If you have specific policy suggestions related to these topics, we encourage you to submit your ideas through the Day One Open Call at FAS.
Pillar 6. Mobilize the Collective Strengths of our Allies and Partners
Pillar 6 of the final report focuses on strengthening alliances and partnerships on the global stage to enhance the U.S. biotechnology sector. It highlights the role of the State Department (DOS) in facilitating this effort through development of foreign policy tools, strengthening global data and market infrastructure, and leading in the establishment of international standards within the sector. Elevating biotechnology within U.S. foreign policy is both timely and necessary, particularly as biotech becomes increasingly strategic in areas like health security, climate resilience, and defense. Leveraging existing tools like the International Technology Security and Innovation (ITSI) Fund could provide a solid foundation, but effective execution will require clearer interagency coordination, transparency in funding allocation, and defined metrics for impact, especially across overlapping technology domains.
Proposals to create shared data infrastructure, joint purchasing mechanisms, and international fellowships point to smart long-term strategies for building trust and interoperability with allies. Yet, success hinges on careful coordination, especially around sensitive areas like dual-use biotechnology export controls. If U.S. standards are significantly more restrictive than those of allies, it could create friction and undermine broader goals of international collaboration and leadership.
FAS provided several additional recommendations and insights on these topics (see Table 2) to make them more nuanced and actionable by Congress, including:
- A Matter of Trust: Helping the Bioeconomy Reach Its Full Potential with Translational Governance by Christopher J. Gillespie
- Strategic Investments the U.S. Should Make in the Bioeconomy Right Now by Nazish Jeffery
- Strengthening U.S. Engagement in International Standards Bodies by Natalie Thompson and Mark Montgomery
- What’s Next for the U.S. Bioeconomy? Defining It. by Nazish Jeffery
One particularly important recommendation emphasizes the need to engage the public and build trust in the sector by collecting data on public acceptance. This data can help inform national governance and ensure it is more responsive and translatable to public concerns.
Additional Considerations
The additional considerations section of the NSCEB report brings several key recommendations that do not fit with the rest of the report, though are still very important. Many focus on aligning federal leadership and economic infrastructure with the needs of a growing and strategically vital biotechnology sector. Elevating biotechnology leadership within DOD is a logical step to align R&D with budget authority and operational needs. Similarly, expanding the scope of the Bioenergy Technologies Office (BETO) beyond biofuels and codifying the Office of Critical and Emerging Technology (OCET) role reflects an overdue shift toward recognizing biotech’s relevance to national security and broader innovation policy, though these changes will require institutional buy-in and cultural adjustment. On the economic side, proposals to create a public-private innovation consortium are timely, especially for supporting smaller firms and navigating the convergence of biotechnology with other technologies, like AI. However, care should be taken to not overly narrow the scope at the expense of other critical intersections.
While FAS provided a few recommendations on these topics (see Table 2), such as:
- Summary Report – December 5, 2022, Bioeconomy Policy Workshop: Measurement and Language
- Laying the Groundwork for the Bioeconomy by Sarah Carter
One of the report’s recommendations would benefit from additional stakeholder input to enhance clarity and ensure that it is actionable for Congress:
- Recommendation 8: Congress should direct the National Science Foundation (NSF) to establish a federal grant program for a national system of community biology labs that would engage Americans in informal learning. Specifically, stakeholder input on:
- What is most needed to support community biology labs?
- Should community labs be incorporated within universities or run as independent institutions?
If you have specific policy suggestions related to these topics, we encourage you to submit your ideas through the Day One Open Call at FAS.
Next Steps for the U.S. Bioeconomy
The NSCEB’s final report outlines a vision for a national biotechnology strategy aimed at securing U.S. leadership in a sector that is not only rapidly advancing but also delivering significant economic returns, outpacing even AI. While the report offers thoughtful, well-grounded recommendations that address many of the core challenges facing the U.S. biotechnology landscape, several proposals would benefit from greater specificity and refinement to make them actionable in legislative form. This moment presents a unique opportunity for stakeholders across the biotechnology ecosystem to contribute meaningfully to the development of a national bioeconomy strategy.
The U.S. bioeconomy, which encompasses biotechnology, holds enormous strategic and economic potential. Without a clear, well-implemented plan, the nation risks repeating the mistakes of past industrial shifts, such as the decline in domestic semiconductor leadership. FAS urges Congress to act on the Commission’s recommendations and leverage FAS’ additional proposals to strengthen them further (see Table 2). We also call on the scientific community to provide additional input on these recommendations to ensure they are viable and impactful. If you have actionable policy ideas on how to shape the path forward for the U.S. bioeconomy, we encourage you to submit them through the Day One Open Call. Applicants with compelling ideas will be partnered with our team at FAS to develop their idea into an implementation ready policy memo. Click here to learn more about the Day One Open Call.
FAS Bioeconomy Open Call Areas
- Recommendation 2.2d (SBIR and STTR): Congress should improve the effectiveness and reach of the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs to support early-stage innovation.
- Which areas of biotechnology or sectors within the bioeconomy would most benefit from SBIR and STTR investment?
- How can these programs better support not only early- but also late-stage innovation?
- Recommendation 4.1c (Bio Data Generation): Congress should authorize and fund the Department of Interior to create a Sequencing Public Lands Initiative to collect new data from U.S. public lands that researchers can use to drive innovation and Recommendation 4.1d: Congress should authorize the National Science Foundation to establish a network of “cloud labs,” giving researchers state-of-the-art tools to make data generation easier.
- What type of data should be generated and what types of data generation or collection should be prioritized?
- How can we best draw on or expand existing cloud lab capabilities?
- Recommendation 4.3b (Predictable BioEngineering): Congress should initiate a grand research challenge focused on making biotechnology predictably engineerable.
- What specific grand challenge should Congress pursue and how should it be implemented?
- How should the U.S. government engage the scientific community (and others) in establishing and pursuing grand challenges for biotechnology?
- Recommendation 4.4a (Safe, Secure, Responsible): Congress must direct the executive branch to advance safe, secure, and responsible biotechnology research and innovation.
- The report calls for establishment of a body within the U.S. government for this purpose. What should this look like and how would it operate?
- Recommendation 5.2a (Domestic Bio Workforce): Congress must maximize the impact of domestic biomanufacturing workforce training programs.
- How should the government approach creating competency models for biomanufacturing training and microcredentialing?
- Which specific areas are best suited for microcredentialing efforts?
- Additional Considerations #8 (Grant Programs): Congress should direct the National Science Foundation (NSF) to establish a federal grant program for a national system of community biology labs that would engage Americans in informal learning.
- What is most needed to support community biology labs?
- Should community labs be incorporated within universities or run as independent institutions?
De-Risking the U.S. Bioeconomy by Establishing Financial Mechanisms to Drive Growth and Innovation
The bioeconomy is a pivotal economic sector driving national growth, technological innovation, and global competitiveness. However, the biotechnology innovation and biomanufacturing sector faces significant challenges, particularly in scaling technologies and overcoming long development timelines that don’t align with short-term return expectations from investors. These extended timelines and the inherent risks involved lead to funding gaps that hinder the successful commercialization of technologies and bio-based products. If obstacles like the ‘Valleys of Death, a lack of capital at crucial development junctures, that companies and technology struggle to overcome are not addressed, this could result in economic stagnation and the U.S. losing its competitive edge in the global bioeconomy.
Government programs like SBIR and STTR lessen the financial gap inherent in the U.S. bioeconomy, but existing financial mechanisms have proven insufficient to fully de-risk the sector and attract the necessary private investment. In FY24, the National Defense Authorization Act established the Office of Strategic Capital within the Department of Defense to provide financial and technical support for its 31 ‘Covered Technology Categories’, which includes biotechnology and biomanufacturing. To address the challenges associated with de-risking biotechnology and biomanufacturing within the U.S. bioeconomy, the Office of Strategic Capital within the Department of Defense should house a Bioeconomy Finance Program. This program would offer tailored financial incentives such as loans, tax credits, and volume guarantees, targeting both short-term and long-term scale-up needs in biomanufacturing and biotechnology.
By providing these essential funding mechanisms, the Bioeconomy Finance Program will reduce the risks inherent in biotechnology innovation, encouraging more private sector investment. In parallel, states and regions across the country should develop regional specific strategies, like investing in necessary infrastructure, and fostering public-private partnerships, to complement the federal government’s initiatives to de-risk the sector. Together, these coordinated efforts will create a sustainable, competitive bioeconomy that supports economic growth, and strengthens U.S. national security.
Challenge & Opportunity
The U.S. bioeconomy encompasses economic activity derived from the life sciences, particularly in biotechnology and biomanufacturing. The sector plays an important role in driving national growth and innovation. Given its broad reach across industries, impact on job creation, potential for technological advancements, and requirement for global competitiveness, the U.S. bioeconomy is a critical sector for U.S. policymakers to support. With continued development and growth, the U.S. bioeconomy promises not only economic benefits, but also strengthens national security, health outcomes, and environmental sustainability for the country.
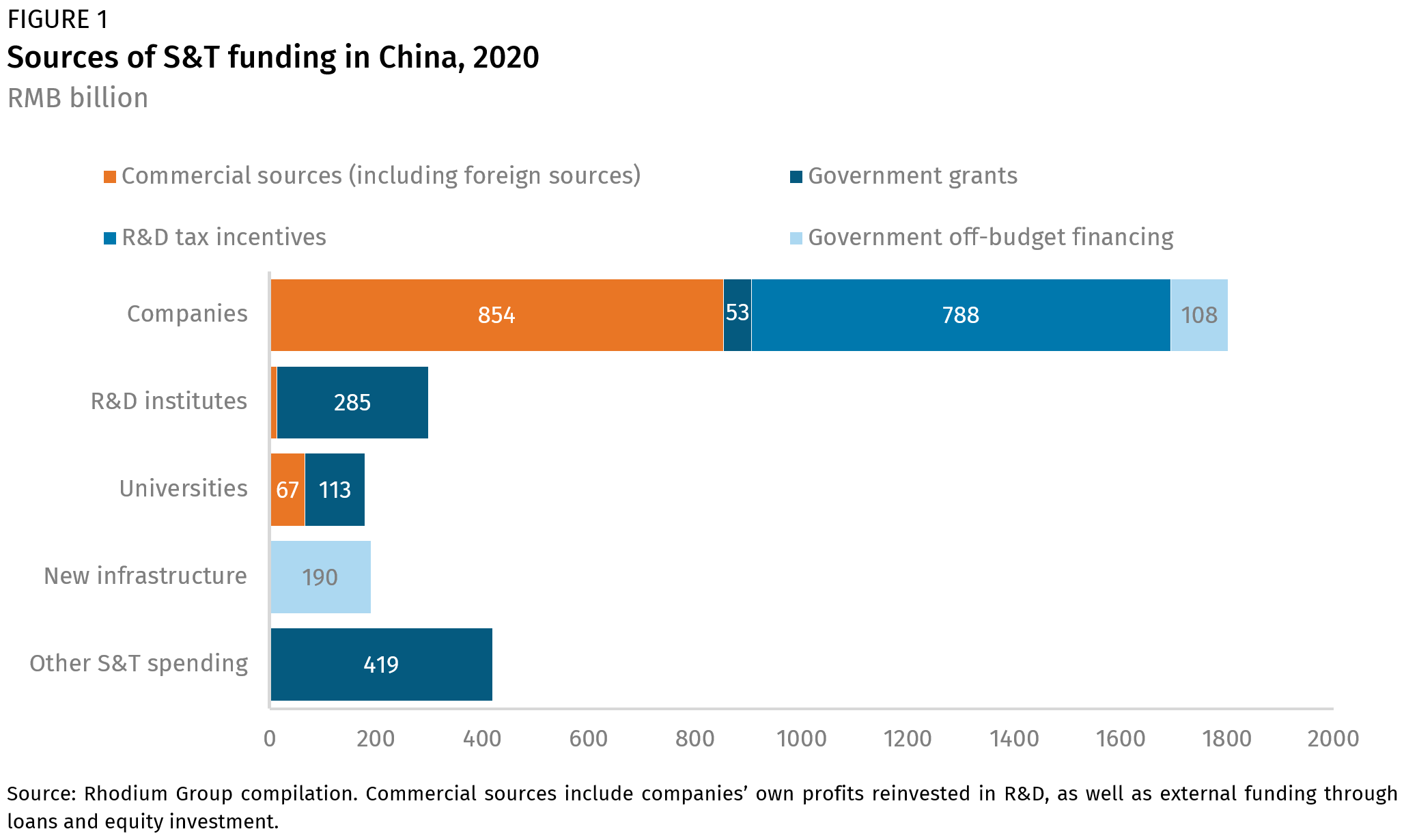
Ongoing advancements in biotechnology, including artificial intelligence and automation, have accelerated the growth of the bioeconomy, making the sector both globally competitive and an important domestic economic sector. In 2023, the U.S. bioeconomy supported nearly 644,000 domestic jobs, contributed $210 billion to the GDP, and generated $49 billion in wages. Biomanufactured products within the bioeconomy span multiple categories (Figure 1). Growth here will drive future economic development and address societal challenges, making the bioeconomy a key priority for government investment and strategic focus.
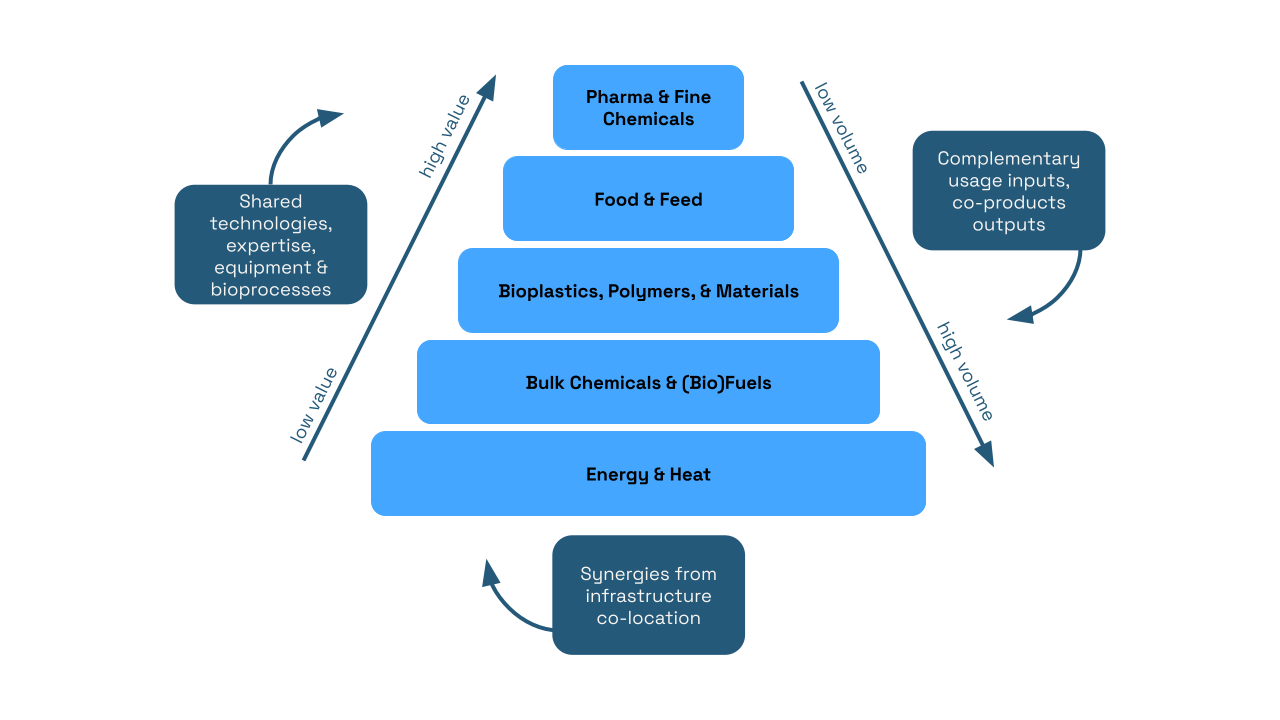
Biomanufactured products span a wide range of categories, from pharmaceuticals and chemicals, which require small volumes of biomass but yield high-value products, to energy and heat, which require larger volumes of biomass but result in lower-value products. Additionally, there are common infrastructure synergies, bioprocesses, and complementary input-output relationships that facilitate a circular bioeconomy within bioproduct manufacturing. Source: https://edepot.wur.nl/407896
An important driving force for the U.S. bioeconomy is biotechnology and biomanufacturing innovation. However, bringing biotechnologies to market requires substantial investment, capital, and most importantly, time. Unlike other technology sectors which see returns on investment within a short period of time, often, there is a misalignment between scientific and capitalistic expectations. Many biotechnology based companies rely on venture capital, a form of private equity investments, to finance their operations. However, venture capitalists (VCs) typically operate on short return on investment timelines, which may not align with the longer development cycles characteristic of the biotechnology sector (Figure 2). Additionally, the need for large-scale and the high capital expenditures (CAPEX) required for commercially profitable production, along with the low-profit margins in high-volume commodity production, create further barriers to obtaining investment. While this misalignment is not universal, it remains a challenge for many biotech startups.
The U.S. government has implemented several programs to address the financing void that often arises during the biotechnology innovation process. These include the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs, which provide phased funding across all Technology Readiness Levels (TRLs); the DOE Loan Program Office, which offers debt financing for energy-related innovations; the DOE Office of Clean Energy Demonstrations which provides funding for demonstration-scale projects that provide proof of concept; and the newly established Office of Strategic Capital (OSC) within the DOD (as outlined in the FY24 National Defense Authorization Act), which is tasked with issuing loans and loan guarantees to stimulate private investment in critical technologies. An example is the office’s new Equipment Loan Financing through OSC’s Credit Program.
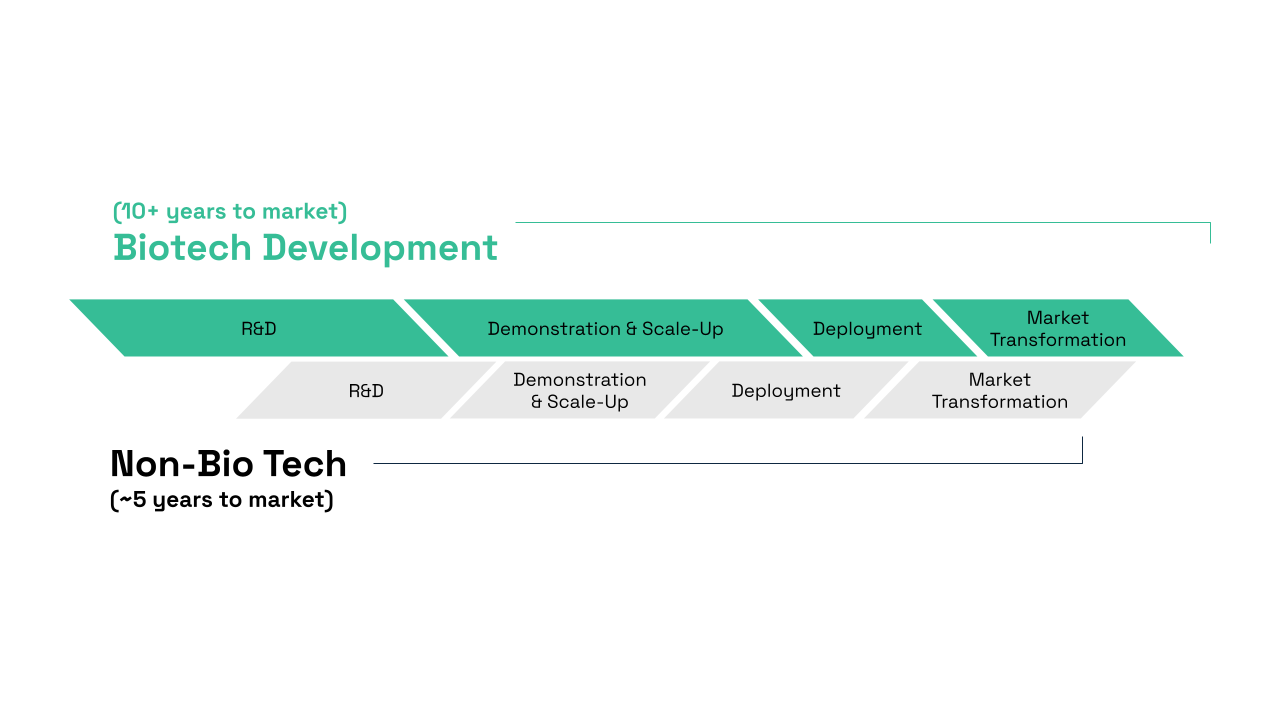
Biotechnology development timelines typically take around ~10+ years to complete and reach the market due to longer R&D and Demonstration & Scale-Up phases, while non-biotechnology development timelines are generally much shorter, averaging around ~5+ years.
While these efforts are important, they are insufficient on their own to de-risk the sector to the degree which is needed to realize the full potential of the U.S. bioeconomy. To effectively support the biotechnology innovation pipeline at critical stages, the government must explore and implement additional financial mechanisms that attract more private investment and mitigate the inherent risks associated with biotechnology innovation. Building on existing resources like the Regional Technology and Innovation Hubs, NSF Regional Innovation Engines, and Manufacturing USA Institutes, help stimulate private sector investment and are crucial for strengthening the nation’s economic competitiveness.
The newly established Office of Strategic Capital (OSC) within the DOD is well-positioned to enhance resilience in critical sectors for national security, including biotechnology and biomanufacturing, through large-scale investments. Biotechnology and biomanufacturing inherently require significant CAPEX, expenses related to the purchase, upgrade, or maintenance of physical assets. This requires substantial amounts of strategic and concessional capital to de-risk and accelerate the biomanufacturing process. By creating, implementing, and leveraging various financial incentives and resources, the Office of Strategic Capital can help build the robust infrastructure necessary for private sector engagement.
To achieve this, the U.S. government should create the Bioeconomy Finance Program (BFP) within the OSC, specifically tasked with enabling and de-risking the biotechnology and biomanufacturing sectors through financial incentives and programs. The BFP should focus on different levels of funding based on the time required to scale, addressing potential ‘Valleys of Death’ that occur during the biomanufacturing and biotechnology innovation process. These funding levels would target short-term (1-2 years) scale-up hurdles to accelerate the biotechnology and biomanufacturing process, as well as long-term (3-5 years) scale-up challenges, providing transformative funding mechanisms that could either make or break entire sectors.
In addition to the federal programs within the BFP to de-risk the sector, states and regions must also make substantial investments and collaborate with federal efforts to accelerate biomanufacturing and biotechnology ecosystems within their own areas. While the federal government can provide a top-down strategy, regional efforts are critical for supporting the sector with bottom-up strategies that complement and align with federal investments and programs, ultimately enabling a sustainable and competitive biotechnology and biomanufacturing industry regionally. To facilitate this, regions should develop and implement state-wide investment initiatives like resource analysis, infrastructure programs, and a cohesive, long-term strategy focused on public-private partnerships. The federal government can encourage these regional efforts by ensuring continued funding for biotechnology hubs and creating additional opportunities for federal investment in the future.
Plan of Action
To strengthen and increase the competitiveness of the U.S. bioeconomy, a coordinated approach is needed that combines federal leadership with state-level action. This includes establishing a dedicated Bioeconomy Finance Program within the Office of Strategic Capital to create targeted financial mechanisms, such as loan programs, tax incentives, and volume guarantees. Additionally, states must be empowered to support commercial-scale biomanufacturing and infrastructure development, leveraging tech hubs, cross-regional partnerships, and building public-private partnerships to build capacity and foster innovation nationwide.
Recommendation 1. Establish and Fund a Bioeconomy Finance Program
Congress, in the next National Defense Authorization Act, should codify the Office of Strategic Capital (OSC) within DOD and authorize the creation of a Bioeconomy Finance Program (BFP) within the OSC to provide centralized federal structure for addressing financial gaps in the bioeconomy, thereby increasing productivity and competitiveness globally. In 2024, Congress expanded the OSCs mission to offer financial and technical support to entities within its 31 ‘Covered Technology Categories,’ including biotechnology and biomanufacturing. Additionally, in order to build resilience in the sector and maintain a competitive advantage globally while also strengthening national security, these substantial expenditures should be housed within the OSC. Establishing the BFP within the OSC at the DOD would allow for a targeted focus on these critical sectors, ensuring long-term stability and resilience against political shifts.
The DOD and OSC should leverage its own funding as well as its existing partnership with the Small Business Administration to direct $1 billion to set up the BFP to create and implement initiatives aimed at de-risking the U.S. bioeconomy. The Bioeconomy Finance Program should work closely with relevant federal agencies, such as the DOE, Department of Agriculture (USDA), and the Department of Commerce (DOC), to ensure a long-term cohesive strategy for financing bioeconomy innovation and biomanufacturing capacity.
Recommendation 2. Task the Bioeconomy Finance Program with Key Initiatives
A key element of the OSC’s mission and investment strategy is to provide financial incentives and support to entities within its 31 ‘Core Technology Categories’. By having BFP design and manage these financial initiatives for the biotechnology and biomanufacturing sectors, the OSC can leverage lessons from similar programs, such as the DOE’s loan program, to address the unique needs of these critical industries, which are essential for national security and economic growth.
Currently, the OSC has launched a credit program for equipment financing. While this is a necessary first step in fulfilling the office’s mission, the program is open to all 31 ‘Core Technology Categories’, resulting in broad, dilutive funding. To accelerate the bioeconomy and reduce risks in biotechnology and biomanufacturing, it is crucial to allocate resources specifically to these sectors. Therefore, BFP should take the lead in several key financial initiatives to support the growth of the bioeconomy, including:
Loan Programs
The BFP should develop specific biotechnology enabling loan programs, in addition to the new equipment loan financing program run by the OSC. These loan programs should be modeled after those in the DOE LPO, focusing on biomanufacturing scale-up, technology transfer, and overcoming financing gaps that hinder commercialization.
Example loan programs:
- DOE Title 17 Clean Energy Financing Program
- USDA Business & Industry Loan Guarantee
- Solar Foods EU Grant/Loan
Tax Incentives
The BFP office should create tax incentives tailored to the bioeconomy, such as, transferable investment and production tax credits. For example, the 45V tax credit for production of clean hydrogen could serve as a model for similar incentives aimed at other bioproducts.
Example tax incentives:
- The Inflation Reduction Act’s transferable tax credits are the gold standard for this category.
Volume Guarantees & Procurement Support
To mitigate risks in biomanufacturing, the office should establish volume guarantees for various bioproducts, offering financial assurance to manufacturers and encouraging private sector investment. An initial assessment should be conducted to identify which bioproducts are best suited for such guarantees. Additionally, the office should explore the possibility of procurement programs to increase government demand for bio-based products, further incentivizing industry growth and innovation. This effort should be undertaken in coordination with the USDA’s BioPreferred Program to minimize redundancy and to create a cohesive procurement strategy. In addition, the BFP should look to the procurement innovations promoted by the Office of Federal Procurement Policy to find solutions for forward funding to create a functioning market.
Example Volume Guarantees & Procurement Support:
- Heavy Forging Press Infrastructure Lease Agreement
- NASA and USAF buying Fairchild semiconductors in advance of needing them, and overbought performance
- Advance Market Commitments
- Joint Venture Partnerships
- Other Transaction Authorities
Recommendation 3. Develop Pipeline Programs to Address Financial and Time Horizon Needs
Utilizing the key initiatives highlighted above, the BFP should create a two-tiered financial mechanisms pipeline and program to address both the short-term and long-term financial needs. The different financial levels could potentially include:
- Level 1 – Short Term Scale-Up (1-2 years) Programs
- Subsidized cost of electricity and other utilities (waste, wastewater treatment, natural gas, energy, etc.)
- Funding for demonstration-scale projects and early-stage engineering development. Similar to the DOEs Office of Clean Energy Demonstrations or the DODs’ Defense Industrial Base Consortium round one $1-2M engineering grants)
- Tax holidays for corporate taxes and property taxes
- Allowing accelerated depreciation to reduce tax liabilities
- Land grants or subsidies for manufacturing assets
- Fast-track permitting and site preparation to avoid long waits
- Labor and workforce subsidies
- Removal of export duties on products created in the U.S. and shipped overseas
- Level 2 – Long Term Scale-Up (3-5 years) Programs
- Large-scale transferable tax credits (either production or investment tax credits) for manufacturing. Similar to the tax credits seen in the Inflation Reduction Act for clean energy.
- Large-scale manufacturing grants
- Large-scale, low-interest manufacturing loans and loan guarantees
- Government procurement contracts or commitment for offtake, such as partial/full volume guarantees
- Government direct or indirect equity investments in biomanufacturing and biotechnology innovations
Recommendation 4. State-Level Initiatives, Infrastructure Development, and Public-Private Partnerships
While federal efforts are crucial, a bottom-up approach is needed to support biomanufacturing and the bioeconomy at the state level. The federal government can support these regional activities by providing targeted funding, policy guidance, and financial incentives that align with regional priorities, ensuring a coordinated effort toward industry growth. States should be encouraged to complement federal initiatives by developing programs that support commercial-scale biomanufacturing. Key actions include:
- State-Level Bioeconomy Resource Analysis: Each state and region should conduct their own analysis to understand the bioeconomy resources at their disposal and determine what relevant resources they would need to establish or strengthen state or regional bioeconomies. Identifying these resources will help the nation understand its true bioeconomic potential by understanding where certain biomass is contained, what facilities are available and needed to develop an economically sustainable bioeconomy, and create data to better understand the economic return on investment.
- Once the analysis is completed, States should collaborate with federal agencies like the DOE, DOC, and Economic Development Administration (EDA) to create and apply for specialized grants for commercial-scale biomanufacturing facilities based off of these analyses. Grants should prioritize non-pharmaceutical biomanufacturing to expand the scope of bioeconomy growth beyond traditional sectors.
- Utility Infrastructure Grants: Another critical area is the creation of utility infrastructure needed to support biomanufacturing, such as wastewater treatment and electricity infrastructure. States should receive targeted funding for these infrastructure projects, which are essential for scaling up production. States should take these targeted funds and establish their own granting mechanism to build necessary, regional infrastructure that is needed long-term to support the U.S. bioeconomy.
- Tech Hub Partnerships: States should leverage existing tech hubs to serve as centers for innovation in bioeconomy technologies. These hubs, which are already positioned in regions with high technological readiness, can be incentivized to partner with other regions that may not yet have robust tech ecosystems. The goal is to create a collaborative, cross-regional network that fosters knowledge-sharing and builds capacity across the country.
- Foster Public-Private Partnerships (PPP): To ensure the success and sustainability of these initiatives, states should actively foster PPPs that bring together government, industry leaders, and academic institutions. These partnerships can help align private sector investment with public goals, enhance resource sharing, and accelerate the commercialization of bioeconomy technologies. By engaging in collaborative R&D, sharing infrastructure costs, and co-developing new biotechnologies, PPPs will play a crucial role in driving innovation and economic growth in the bioeconomy sector. In addition to fostering PPPs, regions should proactively work on creating models that enable these partnerships to become self-sustaining, helping to mitigate potential financial pitfalls if partners drop out of the partnership. By not only creating PPPs, but also ensuring they become fully independent over time, the associated risks with PPPs decrease significantly.
By addressing these steps at both the federal and state levels, the U.S. can create a robust, scalable framework for financing biomanufacturing and the broader bioeconomy, supporting the transition from early-stage innovation to commercial success and ensuring long-term economic competitiveness. A good example of how this approach works is the DOE Loan Program Office, which collaborates with state energy financing institutions. This partnership has successfully supported various projects by leveraging both federal and state resources to accelerate innovation and drive economic growth. This model makes sense for biomanufacturing and biotechnology within the BFP in the OSC, as it ensures coordination between federal and state efforts, de-risks the sector, and facilitates the scaling of transformative technologies.
Conclusion
Biotechnology innovation and biomanufacturing are critical components of the U.S. bioeconomy which drives innovation, economic growth, and global competitiveness, but these sectors face significant challenges due to the misalignment of development timelines and investment cycles. The sector’s inherent risks and long development processes create funding gaps, hindering the commercialization of vital biotechnologies and products. These challenges, including the ‘Valleys of Death,’ could stifle innovation, slow down progress, and result in the U.S. losing its global leadership in biotechnology if left unaddressed.
To overcome these obstacles, a coordinated and comprehensive approach to de-risk the sector is necessary. The establishment of the Bioeconomy Finance Program (BFP) within the DOD’s Office of Strategic Capital (OSC) offers a robust solution by providing targeted financial incentives, such as loans, tax credits, and volume guarantees, designed to de-risk the sector and attract private investment. These financial mechanisms would address both short-term and long-term scale-up needs, helping to bridge funding gaps and accelerate the transition from innovation to commercialization. Furthermore, building on existing government resources, alongside fostering state-level initiatives such as infrastructure development, and public-private partnerships, will create a holistic ecosystem that supports biotechnology and biomanufacturing at every stage and will substantially de-risk the sector. By empowering regions to develop their own bioeconomy strategies and leverage local federal government programs, like the EDA Tech Hubs, the U.S. can create a sustainable, scalable framework for growth. By taking these steps, the U.S. can strengthen both its economic position but also lead the world in development of transformative biotechnologies.
BioMADE, a Manufacturing Innovation Institute sponsored by the U.S. Department of Defense, plays an important role in advancing and developing the U.S. bioeconomy. Yet, BioMADE currently funds pilot to intermediate-scale projects, rather than commercial-scale projects. This leaves a significant funding gap, creating a distinct and significant challenge for the bioeconomy.. By contrast, the BFP within OSC would complement existing efforts by specifically targeting and mitigating risks in the biotechnology and biomanufacturing pipeline that current programs do not address. Furthermore, given that BioMADE is also funded by the DOD, enhanced coordination between these programs willenable a more robust and cohesive strategy to accelerate the growth of the U.S. bioeconomy.
While Private-Public Partnerships (PPPs) are already embedded in some federal regional programs, such as the EDA Tech Hubs, not all states or regions have access to these initiatives or funding. To ensure equitable growth and fully harness the economic potential of the bioeconomy across the nation, it will be important for regions and states to actively seek additional partnerships beyond federally-driven programs. This will empower them to build their own regional bioeconomies, or microbioeconomies, by tapping into regional strengths, resources, and expertise to drive localized innovation. Moreover, federal programs like EDA Tech Hubs are often focused on advancing existing technologies, rather than fostering the development of new ones. By expanding PPPs across the biotech sector, states and regions can spur broader economic growth and innovation by holistically developing all areas of biotechnology and biomanufacturing, enhancing the overall bioeconomy.
Strategic Investments the U.S. Should Make in the Bioeconomy Right Now
In 2023, the U.S. bioeconomy generated 643,992 domestic jobs and contributed $210.4 billion to the U.S. GDP, establishing it as a significant economic force. This impact is largely due to its broad and diverse scope. While the U.S. bioeconomy does not have a consensus definition, nearly all versions of the definition include biotechnology as a central driver. Consequently, a wide range of industries are encompassed within the bioeconomy (Table 1). Previous administrations, including the previous Trump Administration, have championed and advanced biotechnology and biomanufacturing. The Biden Administration released the Executive Order on Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy (Bioeconomy EO) focused on expanding domestic biomanufacturing capacity, streamlining regulations for biotech products, and expanding market opportunities. With a new chapter ahead of us, the United States is presented with incredible challenges and opportunities in the face of China’s dominance in this space.
However, the broad scope of the U.S. bioeconomy also presents significant challenges. While there is general consensus on the types of industries relevant to the bioeconomy, there is no definitive agreement on which should be included or excluded, resulting in certain sectors within an industry being classified as part of the bioeconomy even if the industry as a whole is not. This lack of clarity creates confusion which could result in missed opportunities for ongoing development and support programs that these industries could benefit from. Furthermore, this ambiguity fosters the creation of artificial silos, hindering cross-sector communication and potentially causing a loss of valuable collaborative capabilities. This lack of clarity additionally complicates the measurement of the bioeconomy’s economic contributions, leaving valuable opportunities for growth and development underexplored due to an inability to identify sectors that require further investment.
The Bioeconomy EO made significant strides in understanding the U.S. bioeconomy, but substantial growth remains. A key lesson learned is the need for a common, consensus-based definition and lexicon to improve communication across sectors, as agencies like the DOD, USDA, and DOE often struggle to talk across sectors without a shared language. NIST’s Bioeconomy Lexicon, last updated in February 2025, remains incomplete, with important terms like continuous fermentation still undefined. Additionally, there is a growing need for standards and metrics, and continued investment in NIST’s initiatives, which are essential to build a unified strategy that maximizes the bioeconomy’s full potential.
Despite these difficulties, the U.S. bioeconomy continues to demonstrate resilience and growth, particularly as we enter the next technological revolution driven by advancements in artificial intelligence, biotechnology, advanced manufacturing, and sustainable development. This underscores the critical need for investment across all levels of the U.S. bioeconomy. By making strategic investments in the bioeconomy now, the U.S. can position itself to capitalize on future innovations and advancements, unlocking the projected growth potential, which is expected to reach $400 billion by 2030. Investment in the U.S. bioeconomy not only promotes economic growth but also yields broader benefits, like fostering job creation, driving development of new technologies that enhance the quality of life, providing resilience to long-term prosperity, and staking out significant competitive advantage at the global scale. Overall, the U.S. bioeconomy represents a powerful force with immense potential, one that must be recognized and leveraged further.
Investment in the Bioeconomy Drives Regional Development
Given the vastness of the U.S. bioeconomy, a top-down approach alone will not be enough to drive the rapid growth needed for global competitiveness. A bottom-up approach, led by regional efforts, is essential for fostering growth and innovation across the country. Combining both approaches can boost the national GDP, stimulate regional economies, and create jobs. Regional programs like the EDA Tech Hubs, NSF Biofoundries, and U.S. Manufacturing Institutes like BioMADE and NIIMBL are already supporting localized bioeconomies, or micro-bioeconomies, tailored to specific resources and technologies.
While federal initiatives are important, it is equally important to assess their effectiveness. Understanding the return on investment from these programs is essential, not just for local micro-bioeconomies, but for their broader impact on the U.S. economy. For example, BioMADE has seen significant budget increases, including a $450 million boost in 2023, but its 2024 allocation of $75 million across 65 projects raises questions about fund distribution. As BioMADE supports more projects, further evaluation is needed to determine how effectively it is utilizing resources to advance U.S. biotechnology and biomanufacturing.
Ultimately, it will be essential for stakeholders in the U.S. bioeconomy to closely examine regional programs and assess their effectiveness. A comparative analysis of funding across different programs, agencies, and performance metrics will be necessary to ensure these investments are delivering tangible benefits and are aligned with broader bioeconomy goals. One key lesson from the Bioeconomy EO is that the U.S. must acknowledge that we can do more and that we have not yet done enough. The U.S. bioeconomy is still in its early stages, with significant room for growth and improvement. If the federal government and regional entities do not continue investing in this crucial sector, the nation will face serious economic, social, and global challenges in the future. Failing to act will only stifle progress and allow global competitors to surpass the U.S. in production, manufacturing, skill development, and resource acquisition. To ensure the U.S. bioeconomy thrives, sustained investment, decisive action, and a unified national and regional strategy are essential.
What Areas of the U.S. Bioeconomy Still Need Development?
The bioeconomy EO focused on many different components of the bioeconomy to grow and foster, but despite the EO’s best efforts, not all areas have developed to the same degree. Several areas still require further development, investment, and strategy to safeguard and grow portions of the U.S. bioeconomy.
One important area is Supply Chain Resiliency. In March 2024, the USDA published a report aimed at creating a more resilient biomass supply chain, in accordance with the deliverable in section 5C of the bioeconomy EO. Biomass is an important component of the U.S. bioeconomy but the bioeconomy supply chain encompasses much more. Resiliency should extend to ensuring that all material and intellectual inputs and outputs in the U.S. bioeconomy are safeguarded against disruptions, such as those experienced during the COVID-19 pandemic, and built to last. The pandemic exemplified our reliance on products made outside of the U.S. to keep our biomanufacturing ecosystem functioning, such as the need for single-use plastics or specific biological inputs for vaccine production. In light of this, achieving true resilience requires building a flexible, global supply chain to ensure access to diverse biological resources and the ability to rapidly adapt to global market demands, rather than relying solely on a U.S.-centric model. Establishing stockpile agreements and treaties will be key to ensuring the bioeconomy’s supply chain can withstand unforeseen challenges.
Another important aspect is Bio-Based Product Procurement. The bioeconomy EO included several deliverables focused on bio-based procurement, such as identifying procurement challenges, producing annual fiscal reports, and creating new procurement programs within different agencies. However, despite the emphasis on these issues, little has been published beyond a USDA report that offers generic recommendations to address challenges. These recommendations, such as measuring the bioeconomy and coordinating carbon intensity labels, fail to address the real difficulties that have hindered bio-based procurement at the federal level.
Additionally, Workforce Development & Bioliteracy is crucial for the success of the U.S. bioeconomy. To fully capitalize on the potential of the bioeconomy, a skilled workforce is required. While the OSTP has released an action plan to boost the bioeconomy workforce, further investment and coordination are necessary to meet the bioeconomy’s needs. The U.S. manufacturing sector has been the backbone of the economy since the industrial revolution, and transitioning to biomanufacturing offers the opportunity to create new jobs and bring substantial economic growth to regions across the country. However, this shift requires significant workforce development reforms, including reassessing immigration policies to ensure that the best global talent is attracted to the U.S., further strengthening the economy.
Strategic Next Steps for the U.S. Bioeconomy and the New Administration
To strategically foster the growth of the U.S. bioeconomy and remain globally competitive, the new administration must not only prioritize the development of currently underdeveloped areas within the bioeconomy but also establish the foundational infrastructure necessary for long-term success. A crucial first step would be establishing a clear and adaptable definition of the U.S. bioeconomy. This would help not only in measuring its progress but also in defining which sectors and sub-sectors fall under its umbrella.
Additionally, the administration should focus on regional development through the creation of micro-bioeconomies, which would diversify and strengthen the national bioeconomy. Implementing a bottom-up approach allows regions to tailor their strategies to local strengths, like existing industries, academic institutions, or workforce capacities, while aligning with broader federal priorities. This could involve reskilling and redeploying existing manufacturing capacity into biomanufacturing, leveraging local resources, talent, and infrastructure. These efforts would not only support economic growth and job creation at the regional level, but also enhance national resilience by decentralizing production and fostering innovation across the nation.
To support this regional strategy, the federal government must provide a comprehensive national framework based on clear goals and achievable metrics. For instance, a target such as increasing the production of bio-based products by 50% by 2050 for domestic consumption could help coordinate efforts across various agencies and set a clear path for growth. Furthermore, the federal government should focus on providing the necessary infrastructure for biotechnology and biomanufacturing development, not only physical infrastructure, such as processing facilities and biomanufacturing plants, but also intangible infrastructure like workforce development, talent enhancement, financial mechanisms, and intellectual property creation. The Trump administration could also lead in advancing bio-based material production and green chemical production through novel biotechnologies. These efforts would not only benefit American consumers by providing sustainable alternatives to essential products but would also strengthen national security and defense positions.
The Trump administration presents significant opportunities to advance the bioeconomy, but it must also carefully navigate the wide-ranging activities the sector encompasses. Policy changes, particularly cuts to funding for scientific research and development, could have unintended consequences that hinder progress. While some repealed executive orders may not have directly affected the sector, reductions in funding for science and education are likely to create ripple effects, such as a shortage of trained workers or disruptions in the supply chain, which could trigger cascading negative impacts. Therefore, thoughtful and strategic decision-making is crucial to ensuring the bioeconomy reaches its full potential.
While the U.S. has made significant advancements and remained a global leader in biotechnology over the past decade, the next four years will be critical in determining whether it can sustain that leadership. According to the National Security Commission on Emerging Biotechnology, China has prioritized biotechnology and, by extension, the bioeconomy for the past 20 years and is rapidly advancing toward dominance in the field unless the U.S. takes decisive action. Meanwhile, the Netherlands recently announced an investment of approximately €1.3 billion to expand its biotechnology sector, with the goal of becoming a global leader by 2040. This growing international investment signals rising global competition, and the U.S. must strengthen its bioeconomy to stay ahead. The Trump administration does not need to start from scratch; it can build on the accomplishments of its first term and the progress made since.
The Emerging Reach of the Bioeconomy
On Tuesday, 4/8/25, the bipartisan National Security Commission on Emerging Biotechnology (NSCEB) released their findings on how the U.S. can support and bolster the emerging bioeconomy sector. This sector, which includes biotechnology and biomanufacturing, is increasingly important to scientists working across disciplines – and will continue to shape the economic fortunes of regions across the country.
FAS looks forward to dissecting, advancing, and advocating for the Commission’s report. FAS has been active and influential in this sector and has worked with various stakeholders and experts to advance evidence-based policy recommendations to boost the U.S. bioeconomy (more below). While the report provides an essential starting point to grow and secure our biotechnology and biomanufacturing enterprise, it will be important to advocate for the recommendations found within it, but also to add and refine recommendations to meet the ever evolving U.S. bioeconomy.
FAS is especially enthusiastic about the recommendations that emphasize prioritizing and advancing biotechnology at the national level, ensuring the U.S. maintains its innovation edge. We also strongly support the recommendations aimed at scaling biotechnologies and biomanufacturing by fostering private sector growth and leveraging various financial mechanisms. These recommendations are crucial in addressing some of the most urgent challenges facing the U.S. bioeconomy and will serve as a vital step toward establishing a dynamic and adaptable national strategy for the sector. See our policy statement for more details.
Cautious and Enthusiastic Interest
While FAS is optimistic of the impact that this report can have, it is also important for FAS to be cautious around national security issues due to our 80 year old legacy. FAS began in response to how new technologies (nuclear) could be used for war (nuclear weapons). Today we remain watchful of technologies with the potential of misuse. FAS team members involved with national security take an understandably cautionary approach. The confluence of technology and access mean that there is risk associated with bio-products, too. “This opportunity must also be balanced with a clear-eyed understanding that increasing economic competition, heating geopolitics, and advancing life sciences capabilities may change how countries and other actors view the utility of globally repugnant capabilities such as biological weapons.” said Yong-Bee Lim, Associate Director of Global Risk at the Federation of American Scientists.
The report details the importance of safeguarding the biotechnology and biomanufacturing enterprise to remain competitive at the global scale, especially with China (recall the recent semiconductor shortages). However, “it’ll be important to balance both innovation capabilities and risk as we work towards ensuring that the U.S. bioeconomy is a priority area for both the Nation and for National Security.” said Nazish Jeffery, Bioeconomy Policy Manager at the Federation of American Scientists.
Bioeconomy Presents Significant Opportunities
Still, FAS continues advocating and promoting this area with great enthusiasm. The nascent bioeconomy is more than just leading edge biology meets computational gains. There are a myriad of scientific, economic, and social benefits to be had by leveraging this new industry.
No one at FAS knows this better than Nazish Jeffery, who spearheaded efforts to understand this moment. For more than two years she has worked with biologists, technologists, policymakers and biotechnology companies to investigate how the U.S. can maintain competitiveness while distributing economic rewards equitably domestically.
FAS sponsored policy sprints, which are open calls for participants in academia and industry to submit and develop actionable policy memos that address a particular issue or sector. The goal is to infuse diverse perspectives and expertise into policy that improves lives for all Americans. Since the start of the Commission’s investigations, FAS has sponsored policy sprints on topics such as the intersection of biology and artificial intelligence, as well as a sprint soliciting ideas to grow the bioeconomy sector itself.
This emerging technology sector brought in $210 billion into the U.S. economy in 2023, and is projected to grow to $400 billion by 2030. The economic potential of the bioeconomy is significant; policymakers should promote and work in partnership with industry to continue development in distributed regions across the U.S. to invigorate innovation and enable job creation. This opportunity must also be balanced with a clear-eyed understanding that increasing economic competition, heating geopolitics, and advancing life sciences capabilities may change how countries and other actors view the utility of globally repugnant capabilities such as biological weapons.
FAS interviewed, worked with, or sought input from numerous academics, technologists, bio-industry leaders, elected representatives, and organizations, to understand the full spectrum of the value chain and push forward the best ideas.
FAS invites you to take a look at what possibilities lay ahead, presented below.
Bioeconomy Policy Sprint
The FAS Bioeconomy Sprint produced actionable policy memos to strengthen the bioeconomy in concert with outside expertise, including:
- A Matter of Trust: Helping the Bioeconomy Reach Its Full Potential with Translational Governance by Christopher Gilliespie
- BioNETWORK: The Internet of Distributed Biomanufacturing by Justin C. Sanchez
- Coordinating the U.S. Government Approach to the Bioeconomy by Sarah R. Carter
- Strengthening the U.S. Biomanufacturing Sector Through Standardization by Chris Stowers
- Accelerating Biomanufacturing and Producing Cost-Effective Amino Acids through a Grand Challenge by Allison Berke
- Project BOoST: A Biomanufacturing Test Facility Network for Bioprocess Optimization, Scaling, and Training by Ed Chung & Charles Fracchia
- Advancing the U.S. Bioindustrial Production Sector by Michael Fisher
- Accelerating Bioindustry Through Research, Innovation, and Translation by Jon Roberts
Bioeconomy x AI Policy Sprint
Artificial intelligence continues to develop exponentially; these recommendations can scale alongside AI and deliver substantial benefits:
- Develop a Screening Framework Guidance for AI-Enabled Automated Labs by Tessa Alexanian
- An Evidence-Based Approach to Identifying and Mitigating Biological Risks From AI-Enabled Biological Tools by Richard Moulange & Sophie Rose
- A Path to Self-governance of AI-Enabled Biology by Oliver Crook
- A Global Compute Cloud to Advance Safe Science and Innovation by Samuel Curtis
- Establish Collaboration Between Developers of Gene Synthesis Screening Tools and AI Tools Trained on Biological Data by Shrestha Rath
- Responsible and Secure AI in Production Agriculture by Jennifer Clarke
Additional Bioeconomy Research
Given the ongoing cost curve declines of compute, increased access to data, and growing interest in this emerging sector, FAS continues to investigate a range of related topics. Some recent work includes:
- Understanding the U.S. Bioeconomy: Agency Perspectives
- The U.S. Bioeconomy Needs Biomass, But What Is It and How Do We Use It?
- The Importance of Standards for the U.S. Bioeconomy & National Security: A Conversation with Congressman Jake Auchincloss
- Bold Goals Require Bold Funding Levels. The FY25 Requests for the U.S. Bioeconomy Fall Short
- Regulations, funding, and knowledge gaps: Challenges and opportunities in bringing agricultural biotechnology to market
- The U.S. Bioeconomy is Not Yet Sustainable. Here’s What Needs to Change.
- Implementing the Bioeconomy Executive Order: Lessons Learned and Future Considerations
- Wins, Gaps, & Looking Forward in the U.S. Bioeconomy
- “The US needs to lean into an old strength”: Maintaining Progress and Growing US Biomanufacturing
FAS will continue to work in this important area. Ongoing work related to the U.S. bioeconomy will be regularly updated here: https://fas.org/initiative/bioeconomy/
Position on National Security Commission on Emerging Biotechnology Final Report: Charting the Future of Biotechnology
The Federation of American Scientists supports the National Security Commission on Emerging Biotechnology’s Final Report and the Recommendations contained within it.
Charting the Future of Biotechnology delivers 49 recommendations to foster the growth of the biotechnology and biomanufacturing sector within the U.S. bioeconomy. Implementing the recommendations outlined in this report will strengthen the U.S. bioeconomy by establishing a unified national strategy that fosters innovation in biotechnology, ensures our continued global competitiveness, and delivers significant economic and societal benefits to the nation.
FAS is particularly excited by these recommendations:
- 1.1a: Establishment of a National Biotechnology Coordination Office
- 2.2a-d: Economic levers to promote scale-up and innovations coming to market
- 2.4a: Biotechnology infrastructure and data to be classified as “critical infrastructure”
- 3.1a: Department of Defense to consult with stakeholders to define principles for ethical use of biotechnology
- 4.3a-c: Centers for Biotechnology
- 5.1a through 5.3b: Biotechnology workforce for the future
- 6.1a-e: Strengthen global U.S. biotechnology efforts through global policy
These recommendations have the potential to address key challenges within the U.S. bioeconomy, including the lack of a coordinated strategy, commercialization barriers, workforce shortages, and supply chain vulnerabilities.
“FAS applauds the NSCEB’s deep investigation of unlocking U.S.-led biotechnology in the Fourth Industrial Revolution. We look forward to bringing FAS’s unique and effective approach of policy entrepreneurship to realize the promise of these capabilities while reducing the risks of misuse,” said Yong-Bee Lim, Associate Director of Global Risk at the Federation of American Scientists.
“The National Commission on Emerging Biotechnology report developed 50 recommendations to address the major challenges currently facing the U.S. bioeconomy: a lack of strategy and coordination across the federal government, difficulties in scaling biotechnology innovations, and the need for a trained workforce for the future. These recommendations aim to de-risk the biotechnology sector, thereby enabling private sector investment in critical biotechnology and biomanufacturing initiatives. Ultimately, these efforts will foster continued growth, secure the U.S. bioeconomy, and lead to the creation of new jobs and further economic growth.”” said Nazish Jeffery, Bioeconomy Policy Manager at the Federation of American Scientists. “It will be important to continue advocating, refining, and adding additional recommendations in order to realize the full value that this report offers.”
For more information contact Nazish Jeffery, FAS Bioeconomy Policy Manager, njeffery@fas.org.
Understanding the U.S. Bioeconomy: Agency Perspectives
The U.S. bioeconomy—defined by the National Institute of Standards and Technology (NIST) as “economic activity derived from the life sciences, particularly in the areas of biotechnology and biomanufacturing, including industries, products, services, and the workforce” and valued by some at ~$1 trillion—has been a major focus of policy development over the past few years. These policy advances include the White House Executive Order on “Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy” (Bioeconomy EO), the CHIPS & Science Act, and the Inflation Reduction Act (IRA). In March 2024, the Office of Science and Technology Policy (OSTP), announced the launch of the National Bioeconomy Board (NBB). The board will “partner across the public and private sectors to advance societal well-being, national security, sustainability, economic productivity, and competitiveness through biotechnology and biomanufacturing,” highlighting the Biden Administration’s commitment to future-proofing an economically sustainable U.S. bioeconomy.
Despite these advances, the vast intersectionality inherent to the bioeconomy (e.g., with health, clean energy, national security, climate change, economic development) poses unique challenges for the U.S. government. This complexity makes it difficult for the various agencies to coordinate and even more difficult for the general public to understand the government’s approach to the bioeconomy. Nonetheless, to maintain the continued growth within the bioeconomy that has resulted from these policy advances, it will be imperative to clarify a strategic vision that coordinates and publicizes governmental efforts that support the burgeoning U.S. bioeconomy.
The NBB can play an important role in promoting this strategic vision. As directed by the Bioeconomy EO, the interagency through the Executive Office of the President set up the NBB to promote interagency coordination and collaboration on the bioeconomy. The NBB is co-chaired by OSTP, the Department of Commerce (DOC), and the Department of Defense (DOD), and nine other agencies make up the entirety of the board. Other agencies not represented on the NBB itself, including the Environmental Protection Agency (EPA), work with the NBB through various working groups and play an integral role.
To understand the range of governmental priorities for the bioeconomy, the overarching strategy, the work underway, the various programs within the agencies, and the role of environmental sustainability, our team at the Federation of American Scientists (FAS) spoke with key agencies represented on the NBB to collect their perspectives.
The perspectives summarized below demonstrate that the agencies align bioeconomy-related initiatives to their varied mission areas and, through the NBB and other interagency activities, are working together to develop a shared vision. However, the summaries also show the diversity in focus that informs how agencies approach the bioeconomy. The agency views encompass the broader bioeconomy landscape, including biotechnologies from commodity fuels and agriculture to individualized therapeutics, and biomanufacturing solutions from biomass production to final product. This range highlights both the important role that each agency plays in supporting the U.S. bioeconomy as well as the challenge in coordinating their activities and programs across the federal government.
Approach
In order to collect perspectives from the agencies represented on the NBB around the U.S. bioeconomy, FAS conducted semi-structured interviews with key NBB officials from the OSTP, DOC, DOD, Department of Energy (DOE), Department of Health and Human Services (HHS), and the U.S. Department of Agriculture (USDA) from May 2024 through June 2024. With the exception of USDA, all agency interviews were conducted over Zoom and answers were documented by note-taking. All summaries have been reviewed by agency representatives to confirm consent and validity. The USDA perspective was summarized using publicly available reports and have also been confirmed for validity by an agency representative.
Perspectives from these agencies on the Bioeconomy EO deliverables, bioeconomy-related programs, coordination, goals, hurdles, and the role of environmental sustainability are summarized below. The full list of questions used in the semi-structured interviews can be found in Appendix A.
Agency Perspectives
Office of Science & Technology Policy
For OSTP’s perspective, FAS conducted a semi-structured interview with Dr. Sarah Glaven, principal assistant director for biotechnology and biomanufacturing.
The Office of Science & Technology Policy plays an important role in interagency coordination for topics, like the bioeconomy, that cut across many different agencies, and is one of the co-chairs for the NBB. In adherence with the Bioeconomy EO, OSTP has coordinated interagency efforts and published several reports on the bioeconomy: Bold Goals for U.S. Biotechnology and Biomanufacturing, Building the Bioworkforce of the Future, and Visions, Needs & Proposed Actions for Data for the Bioeconomy Initiative. They are currently working with interagency groups on several activities, including one that recently published a report, in conjunction with USDA and other agencies, that recommended revisions to the North American Industry Classification System (NAICS) and the North American Product Classification System (NAPCS) to better capture economic activity related to the bioeconomy. The creation of the NBB itself fulfills directives from both the Bioeconomy EO as well as the CHIPS & Science Act, which called on OSTP to establish a coordination office on these topics. Currently, due to a lack of funding, OSTP is not an official coordinating office but will function to coordinate activities through the NBB.
According to OSTP, the Bioeconomy EO reflects the whole-of-government approach that will be needed to support the bioeconomy. For the near term, OSTP plans to show the value and utility of the NBB, execute policy from the Bioeconomy EO, prioritize specific actions from the resulting Bioeconomy EO reports, highlight significant investments, and produce a report on the NAICS and NAPCS codes. In the long term, OSTP hopes that the NBB will become a sustainable government entity that drives a clear national strategy to move the bioeconomy forward and enables the United States to work collaboratively with global partners.
A key challenge is measuring the bioeconomy. It is difficult to prioritize, strategize, or advocate for additional resources in the absence of baseline economic metrics to track impact or estimate the potential return on investment. Ultimately, OSTP believes it is important to clarify the definition of the bioeconomy in order to create measurements and classifications.
A challenge for OSTP is continuity as it experiences staff turnover and administration changes. However, the NBB and coordination of the bioeconomy portfolio will be well positioned to persist, in part by relying on the NBB’s co-chairs. Also, the Bioeconomy EO allowed OSTP to create principal and assistant director positions for the bioeconomy portfolio, which can help ensure that it remains a high priority. At OSTP, this portfolio sits within the Industrial Innovation Group, which also houses coordination efforts for semiconductors and clean energy. OSTP leadership understands the importance of the bioeconomy and is keen to see the intersections of biomanufacturing with other initiatives, like the DOE’s Earth Shot programs and other clean energy initiatives.
On the issue of environmental sustainability and the bioeconomy, OSTP highlights efforts by DOE to push for sustainable aviation fuels and USDA’s sustainable biomass supply chain framework as initiatives that are setting the pace for sustainability. There is also an opportunity to consider how biomanufacturing and biosynthesis fit into the broader sustainable chemistry landscape.
Department of Commerce
For DOC’s perspective, FAS conducted a semi-structured interview with Dr. Christopher Szakal, acting director, program coordination office at the National Institute of Standards and Technology.
The Department of Commerce is one of the NBB co-chairs. DOC is sector-agnostic and is interested in the bioeconomy as a way to support the broader economy, remain competitive, and solve broader challenges, such as those related to supply chain resilience. In response to the Bioeconomy EO, the DOC has released the bioeconomy lexicon from NIST and the Feasibility Study for measuring the bioeconomy from the Bureau of Economic Analysis. It has also participated in several interagency activities, including development of OSTP’s Bold Goals report and USDA’s Biomass Supply Chain report, as well as ongoing working groups focusing on updating systems for measuring economic activities (e.g., the NAICS and NAPCS codes) and on biological data and cybersecurity. Separate from the executive order, the Inflation Reduction Act provided significant investments for the Economic Development Administration in biotechnology-related regional technology hubs. Other ongoing activities at DOC in support of the bioeconomy include efforts to support biotechnology and biomanufacturing standards development at NIST, supply chain analyses at the International Trade Administration, work at the Bureau of Industry and Security and at the Patent and Trademark Office to ensure a safe and fair market, and the Workforce Development Strategy.
By nature, DOC keeps a broad perspective and tries to understand how the bioeconomy intersects with other parts of the economy and how technological developments may impact progress. There are important intersections of the bioeconomy with artificial intelligence (AI) and with data security, and policy development in these other areas will have implications for the bioeconomy. For example, the October 2023 Executive Order on AI called for significant new requirements for providers of synthetic nucleic acids to conduct biosecurity screening, which will have implications for biotechnology and biomanufacturing. NIST is tasked with developing standards for this new policy. The intersectional nature of the bioeconomy requires coordination both within the DOC and across the U.S. government. A key challenge is the need for sustained funding because coordination requires time and effort.
On environmental sustainability, the DOC prioritizes the market and what U.S. companies will find profitable in both the near term and the long term. Elevating sustainability has been challenging because there is uncertainty in how sustainability is measured. Additionally, market drivers have been inconsistent relative to the level needed to address the uncertainty. DOC is looking to utilize the NBB to help provide clarity on how to achieve more consistent market forces in support of sustainability to drive growth of the bioeconomy.
Department of Defense
For DOD’s perspective, FAS conducted a semi-structured interview with Dr. Peter Emanuel, senior research scientist, bioengineering at U.S. Army Combat Capabilities Development Command.
The Department of Defense is one of the NBB co-chairs. In September 2022 (before the Bioeconomy EO was announced), DOD announced a $1.2 billion investment in biomanufacturing. In March 2023, DOD released a Biomanufacturing Strategy, which was informed by both the National Defense Authorization Act for Fiscal Year 2023 and the Bioeconomy EO. In support of this strategy and the investments made by DOD, the Department’s Defense Production Act Investments (DPAI) Office published an open Request for Information that sought input from industry on biomanufactured products and process capabilities that could help address defense needs. Significant additional investments in biomanufacturing are likely to be forthcoming.
The bioeconomy portfolio is a tiny portion of the overall programmatic budget for DOD. Previously, the DOD’s interest in biology and biotechnology was limited to military medicine and chemical and biological defense, but the department is increasingly focused on nonmedical biomanufacturing applications and believes that they will be key for ensuring national security. The department also acknowledges the importance of workforce development and the need for standardization and infrastructure for the bioeconomy and strongly supports these areas. This commitment can be seen with DOD’s large investments in 2020 in BioMADE, a Manufacturing Innovation Institute focused on creating a sustainable, domestic end-to-end bioindustrial manufacturing ecosystem.
In the future, DOD hopes to take advantage of biomanufacturing’s potential to support defense objectives beyond just medical countermeasures and other human health-related advances, such as production of bio-based materials, chemicals, and foods. However, DOD faces challenges both internally and externally in communicating the full potential of the bioeconomy and biomanufacturing for DOD.
On environmental sustainability, DOD believes that economic and environmental sustainability for the bioeconomy go hand-in-hand. For example, a company that could make chemicals without waste would have a significant economic advantage and would support environmental sustainability. Historically, DOD has seen significant costs due to polluted sites, and so understands the value of cleaner products and processes. In addition, DOD is investing in different technologies that would valorize waste streams.
Department of Energy
For DOE’s perspective, FAS conducted a semi-structured interview with Dr. Valerie Reed, director, Bioenergy Technologies Office.
The Department of Energy has many goals for advancing the bioeconomy, with the common denominator being to decarbonize America’s transportation and fuel sectors and to build resilient clean energy for generations to come. In response to the Bioeconomy EO, the DOE contributed to the OSTP Bold Goals report and was tasked to work with other agencies to write reports on National Security Recommendations for Federal Procurement (forthcoming) and best practices for cyber security documentation. Furthermore, DOE also played a large role in an upcoming biotechnology and biomanufacturing report mandated by the Bioeconomy EO. Outside of the direct requirements from the EO, the DOE plays a crucial role in supporting industrial biotechnology through additional reports and their involvement in ongoing interagency activities. For example, the Billion Ton Report provides a comprehensive assessment of biomass availability today and how to sustainably produce more than one billion tons of biomass per year to meet the demand for sustainable aviation fuel production.
DOE’s bioeconomy efforts are concentrated within the Bioenergy Technologies Office (BETO) and the Office of Science. BETO aims to utilize biomass for sustainable and renewable fuel and chemical production, while the Office of Science supports fundamental research that enables the bioeconomy, including synthetic biology and thermochemical conversion. Under the Inflation Reduction Act and CHIPS & Science Act, significant support was given to bioenergy solutions and clean energy demonstrations, including DOE tax incentives aimed at carbon reduction in fuel production.
In the short term, DOE is focused on prioritizing the use of biomass for Sustainable Aviation Fuel (SAF) and marine fuel production, as well as supporting renewable diesel and ethanol for medium- and heavy-duty vehicles. Long-term goals include transitioning to electrification using biomass, achieving substantial SAF production by 2035 through the SAF Grand Challenge, and scaling up the production of specific chemicals by 2035 as part of the industrial decarbonization strategy. Additionally, in coordination with the USDA, there are focused efforts to increase cultivation of purpose-grown energy crops.
One of the major hurdles the DOE currently faces, and may continue to face in the future, is ensuring sustained funding levels that support ongoing development. Currently, biomass is seen as an expensive feedstock. While the IRA provided an initial policy bridge, it is essential to establish a longer-term incentive to meet market demand, like the 40B (SAF production) and 45Z (clean fuel production) tax credits.
On environmental sustainability, the DOE is very focused on goals for decarbonization of transportation and fuels, including replacing petroleum-based products with sustainable biomass solutions and conducting life cycle assessments (LCAs) to measure sustainability impacts throughout the supply chain. DOE created the GREET Model for LCAs, which was updated recently, to reduce ambiguity and to help standardize the process for measuring carbon emissions. Additionally, DOE’s Clean Fuels and Products Earthshot is an important cross-agency collaboration that supports accelerating bio-based fuels and chemicals production and decarbonizing both the fuel and chemical industry.
Department of Health & Human Services
For HHS’s perspective, FAS conducted a semi-structured interview with Dr. Lyric Jorgenson, associate director for science policy and the director of the Office of Science Policy at the National Institutes of Health (NIH), and Dr. Julia Limage, director, Office of Strategy, Policy, and Requirements in the Administration for Strategic Preparedness and Response (ASPR).
The Department of Health & Human Services has two representatives on the NBB, one from NIH and one from ASPR. HHS has a broad mission in support of human health, and many of its programs could be considered part of the bioeconomy. However, the Bioeconomy EO outlined a set of priorities that called for additional focus at HHS on advances specific to biotechnology and biomanufacturing, many of which were included in the OSTP Bold Goals report. The EO also tasked HHS with leading the establishment of a Biosafety and Biosecurity Innovation Initiative; a strategic plan for this initiative will be available soon. Another area of intersection of HHS and the Bioeconomy EO is on the regulatory side: the Food and Drug Administration worked with USDA and EPA to provide updates on the regulatory system as deliverables for the EO. Many of the activities related to the EO draw on interagency working groups and other ongoing activities—for example, the work toward pandemic preparedness and biodefense, as well as collaborations between NIH and NSF on health-relevant research.
In the near future, HHS will focus on advancing biotechnologies such as multi-omic medicine, gene editing, and other therapeutics tailored to individual patients. Biomanufacturing and scale-up is another key focus to increase speed and availability of key medicines. In regards to public health, the COVID pandemic highlighted the need for fast and secure biomanufacturing for vaccine production. The Biomedical Advanced Research and Development Authority (BARDA) in ASPR has made significant investments in biomanufacturing for this reason. ASPR also has an Office of Industrial Base Management and Supply Chain to support domestic biomanufacturing in case of public health emergencies.
For HHS, activities related to the bioeconomy directly and unambiguously support the department’s mission and will continue to be prioritized. A key challenge for HHS is the need for sustained funding, especially for coordination, which requires time and effort above and beyond programmatic work. To be effective, activities initiated by the Bioeconomy EO will need to be funded. Some HHS activities, including some related to biomanufacturing of medical countermeasures, were funded with COVID supplemental funding that will soon run out.
On environmental sustainability, HHS has not had any significant focus. However, there have been efforts to decrease the use of single-use plastics and equipment in research and public health activities.
United States Department of Agriculture
For USDA’s perspective, FAS gathered information from publicly available reports and documents, with guidance and direction from Herrick Fox, USDA’s bioeconomy coordinator in the Office of the Chief Economist, and Greg Jaffe, senior advisor in the Office of the Secretary.
The Bioeconomy EO tasked USDA with a wide range of deliverables, and USDA has released many related reports and products that reflect its bioeconomy-related priorities. One set of deliverables focuses on biomass and feedstocks, and supports the strategic vision outlined for agriculture in OSTP’s Bold Goals report. This includes the report on Building a Resilient Biomass Supply—A Plan to Enable the Bioeconomy in America, along with an Implementation Framework. USDA also has a long-standing focus on bio-based products, including support of the BioPreferred Program, a program created by the 2002 Farm Bill to increase the purchase of bio-based products and reauthorized in the 2018 Farm Bill. Their recent Economic Impact Analysis of the U.S. Biobased Products Industry report summarizes the status of bio-based products, an important component of the bioeconomy.
USDA also plays a central role in regulating biotechnology products, and the Bioeconomy EO called for updates to the regulatory system. In response, USDA (along with the FDA and the EPA) conducted stakeholder outreach, which is summarized in a report on Ambiguities, Gaps, and Uncertainties in Regulation of Biotechnology Under the Coordinated Framework. USDA also released a Plan for Regulatory Reform under the Coordinated Framework for the Regulation of Biotechnology and produced an updated Coordinated Framework website. Activities to improve coordination across the three major regulatory agencies are ongoing.
Unlike most federal departments and agencies, most programs and activities at USDA have a link with the life sciences, including those that support food and fiber, forests and grasslands, and other natural resources, as well as the manufacturing of numerous bio-based products and biofuels from these resources and the R&D and infrastructure that supports it. From USDA’s perspective, the department has served the bioeconomy since its founding in 1862. This broad focus provides many opportunities for strategic partnerships with other parts of the U.S. government working on the bioeconomy, and there are many different ways that USDA can contribute to the NBB.
On environmental sustainability, USDA has demonstrated its commitment to developing a circular bioeconomy, which is reflected in its Biomass Plan, and in its support for bio-based products and sustainable agriculture initiatives.
Conclusion
The agencies that make up the NBB highlight the complex nature of the U.S. bioeconomy and the various sectors that fall under it. Nevertheless, despite this complexity, the NBB is providing a whole-of-government approach to enable agencies to better support the burgeoning U.S. bioeconomy. The work underway is underpinned by the agencies’ priorities and programmatic expertise, but comes together to build the foundational base needed to support and grow the U.S. bioeconomy. Each agency also has a focus on environmental sustainability, with some, like DOE, DOD, and USDA, having a stronger focus due to their direct connections with the environment. Finally, agencies also agree on the need for more data on the bioeconomy’s impact as the different sectors evolve and the need for sustained funding to promote coordination, which takes time and effort beyond just programmatic work.
Appendix A. Interview Questions
- In response to the September 2022 Bioeconomy EO, your agency has produced some reports and other deliverables on the bioeconomy.
- Are we missing any deliverables? Are there any other reports or activities that are already completed or still to come in response to the EO?
- Are there programs or other deliverables relevant to the bioeconomy that your agency has pursued under the Inflation Reduction Act or the CHIPS & Science Act?
- Are there other activities within your agency that you believe support the bioeconomy? Is the bioeconomy broader than what was captured by the EO and these other efforts?
- What does your agency hope to achieve in the foreseeable future and in the more distant future regarding the U.S. bioeconomy?
- Are these goals related to the OSTP Bold Goals Report or other deliverables for the Bioeconomy EO?
- To what extent will this progress be prioritized within your agency? How central to your agency is progress in the bioeconomy – now and into the future?
- What are the major hurdles your agency currently faces or may face in the future in reaching these goals?
- How does your agency tackle the issue of creating an environmentally sustainable bioeconomy and/or a circular bioeconomy?
- Are there any initiatives in place currently or coming up in the near future that speak towards this?
The Importance of Standards for the U.S. Bioeconomy & National Security: A Conversation with Congressman Jake Auchincloss
The U.S. bioeconomy, the sector of the economy that is touched by biology, is valued at ~$1 trillion and predicted to grow to over $30 trillion in the next two decades. With such enormous potential, ensuring the U.S. bioeconomy’s continued economic growth and global leadership has become a matter of importance for national security. Despite this massive potential, the U.S. bioeconomy, and specifically the biomanufacturing industry, is currently limited by the lack of standards in place for the sector.
The need for standards within the biomanufacturing sector has been discussed at length by experts, and the U.S. government has acknowledged and prioritized the establishment of standards by creating a National Standards Strategy for Critical and Emerging Technologies. Both the CHIPS & Science Act of 2022, and the Visions, Needs, and Proposed Actions for the Data for the Bioeconomy Initiative (2023), highlight the need for standardization as critical to the advancement of our domestic biomanufacturing sector. Furthermore, the National Security Commission on Emerging Biotechnology (NSCEB), a commission tasked with reviewing advancements in biotechnology and its nexus with national security, stated in their interim report the importance of creating standards for this sector as a matter of national security.
Most recently, The Select Committee on Strategic Competition between the United States and the Chinese Community Party (Select Committee on the CCP), held a hearing, “Growing Stakes: The Bioeconomy and American National Security”, that focused on the threats posed by adversaries in the industry. During the hearing, Congressman Jake Auchincloss (D-MA, 4th District), entered into the record a bipartisan letter that contained recommendations around developing and implementing standards for the bioeconomy that urged action from the National Institute of Standards and Technology (NIST).
To get a better understanding of how Congress and the Select Committee on the CCP view the need for standards for the bioeconomy, FAS interviewed Congressman Jake Auchincloss.
FAS: In your opinion, why does creating standards for biotechnology and biomanufacturing boost economic competitiveness and national security? What benefits does this pose for different regions across the nation?
Congressman Auchincloss: Standards are important in every industry to solve coordination and collective action problems, and the government plays a critical role in establishing them so that markets can work more effectively. Standards provide concrete benchmarks for individuals and companies within American industry, all of whom need a stable environment to make long-term investments. This sentiment is echoed in the National Security Commission on Emerging Biotechnology’s interim report which has noted that “biomanufacturing faces barriers to innovation because of…lack of standardization.”
With standardization, more time can be spent innovating, researching, and building instead of compensating for uncertainty due to a lack of definitions that carry the weight of the federal government. Establishing standards will only increase productivity. As I stated in the letter I entered into the record during the Select Committee on the CCP’s hearing around the bioeconomy, “standardization will help advance industrial biomanufacturing, create a more resilient and dynamic supply chain, and establish a durable, competitive U.S. bioeconomy. In turn, strengthening the U.S. bioeconomy will improve Americans’ well-being, promote well-paying jobs, and create a competitive and advantageous U.S. science and technology enterprise to achieve our national and societal goals.”
What is the role of international collaboration in creating standards for biotechnology and biomanufacturing in light of increased tensions with China?
International collaboration is critical to the success of biotechnology, but we must ensure we are working with reliable and responsible partners. For the U.S. to remain a leader in the bioeconomy, the U.S. must ensure its domestic standards become the international benchmark.
As we standardize the bioeconomy, we must also set standards for ethical intent and conduct. As such, there are some companies whose loyalty does not side with responsible science. We shouldn’t partner with companies like BGI, whose technologies have clear ties to the CCP’s repression and ongoing ethnic cleansing of its Uyghur communities. The increased tensions are a direct result of President Xi Jinping’s disregard for human rights, and excluding the CCP from projects that could be weaponized against their own people is the correct response.
Where does the U.S. bioeconomy stand in comparison with China or other countries?
China isn’t currently ahead but they are neck-and-neck with the U.S. because of the rate at which they are investing in their domestic bioeconomy. That’s already showing up in their patent and publication volume. The CCP’s R&D investment in biotechnology increased from $26 million USD in 1986 to $99 million USD in 2005. From 2008 to 2020, their investments increased to $3.8 billion USD. China’s spending on research and development overall climbed 10.3 percent to 2.44 trillion Chinese yuan ($378 billion USD) in 2020, according to the nation’s National Bureau of Statistics. Further, according to the health care information company IQVIA, China was the world’s second-largest national biopharmaceutical market in 2017, worth $122.6 billion USD.
The 117th Congress and Biden Administration edged science and technology funding upwards, but Republicans have proposed slashing federal R&D funding. We are moving in the wrong direction with this self-defeating approach. Congress must prioritize basic science: the curiosity-driven, peer-reviewed research that the private sector won’t fund and the public sector under-funds. We can start by expanding NIH funding and fully appropriating the $170 billion Science portion of the Chips and Science law that was authorized throughout the next ten years.
NIST has been directed to create standards and metrology for the U.S. bioeconomy through the FY24 appropriation bills, the FY25 Presidential Budget, the Bioeconomy EO, and from the letter that you submitted into the record during the hearing “Growing Stakes: The Bioeconomy & American Security.” Given all these priorities, what needs to happen in order for NIST to fulfill their directives for the bioeconomy?
There needs to be continued pressure applied to ensure NIST is prioritizing this important work. Congress can further support NIST by appropriating more funding for them to achieve the work laid out in front of them, as I have advocated during the current appropriations process.
The recent AI EO also directs NIST with many different tasks. Is the AI EO overtasking NIST and making bioeconomy related efforts an afterthought for them?
The AI EO can be implemented simultaneously with standardization efforts, if NIST is appropriately resourced. But those who think that AI is more important than biotech are wrong, and should not point NIST in that direction. Care should be taken not to duplicate work unnecessarily, but all of these tasks assigned to NIST will take time and labor. Again, NIST needs to be adequately funded to do all the work it is being asked to do.
In the letter, you suggest NIST collaborate with Manufacturing USA institutes, NIIMBL & BioMADE. However, in the Department of Commerce’s FY25 budget request, they ask for $37M for the Manufacturing USA program, the same amount that they have received since FY23. Should Congress prioritize and raise funding levels for programs like Manufacturing USA and why would this be important for ensuring a competitive edge for the U.S. bioeconomy?
Absolutely. Many federal R&D programs are continuously underfunded, even as they are tasked with more responsibilities. The programs we fund reflect our priorities. We need to be prioritizing science, technology, and centers of excellence for manufacturing to gain that competitive edge. Increasing funding would give these agencies and programs the resources they need to set standards and increase R&D. That’s why I sent a letter to the House Committee on Appropriations asking for a 10 percent increase above FY24 enacted funding levels for NIST, Manufacturing USA, and the Manufacturing Extension Partnership.
Lastly, in your opinion, what potential does the U.S. bioeconomy have that we are not capitalizing on and what would you like to see occur for the U.S. bioeconomy in the next year.
I would like to see standardization, or at least the beginning of standardization, within the next year. With standardization for industrial biomanufacturing in place, the U.S. bioeconomy will be able to reach new heights and enable our talented citizens to delve deeper into their research without being hindered by the lack of baseline definitions. Fully appropriating the $170 billion that was authorized for the Science portion of CHIPS and Science would be the step in the right direction to maintain U.S. innovation and competitiveness in biotechnology and biomanufacturing. Furthermore, we need state capacity and funding for R&D; which includes the staffing and programming for regulation and standardization and also the funding for peer-reviewed basic research. Finally, we need to expand the productive capacity of the bioeconomy through workforce development compacts that bring together employers, educators, and trade associations together; through skilled immigration pathways; and through technology-agnostic tax credits that are transferable for R&D and biomanufacturing. In the next year, I am working towards legislation that advances all of these different components to strengthen and secure the U.S. bioeconomy.
The Federation of American Scientists values diversity of thought and believes that a range of perspectives — informed by evidence — is essential for discourse on scientific and societal issues. Contributors allow us to foster a broader and more inclusive conversation. We encourage constructive discussion around the topics we care about.
The U.S. Bioeconomy needs biomass, but what is it and how do we use it?
In the quest for sustainable energy and materials, biomass emerges as a key player, bridging the gap between the energy sector and the burgeoning U.S. and regional bioeconomies (microbioeconomies). Despite often being pigeonholed as fuel for energy production, biomass holds far-reaching potential that extends beyond combustion. Identifying sustainable biomass feedstocks that are easily accessible and consistent in their makeup could be a game-changer to help regions unlock their bioeconomy potential and support scientific innovations toward more environmentally sustainable materials and chemicals.
Biomass is defined as “any organic matter that is available on a renewable or recurring basis, including agricultural crops and trees, wood and wood residues, plants, algae, grasses, animal manure, municipal residues, and other residue materials” by the Foundation for Food & Agriculture Research (FFAR). Biomass has mainly been viewed by the public as a source of energy through burning or for chemical conversion into biofuels, encouraged by federal incentive programs, including those from the United States Department of Agriculture (USDA) and the Department of Energy (DOE). However, aside from burning or conversion for biofuel, biomass can undergo a complex process of chemical or biological breakdown and be transformed into various building block components that can be used for a wide range of biotechnology applications.
Once the biomass is broken down into its functional components it can be used as a feedstock, which is a “resource used as the basis for manufacturing another product. [Often], . . . a source of carbon to produce an array of chemicals.” For example, lignocellulosic biomass, plant or plant-based materials not used for food, can be hydrolyzed into sugars, which serve as precursors for bio-based chemicals and materials. This allows for new, environmentally sustainable chemicals for use in biotechnology and biomanufacturing applications, thus positioning biomass as a cornerstone resource of the U.S. bioeconomy. In addition to biochemical production, biomass, and feedstock are used in the bioeconomy in bioplastics and biomaterials. To push the U.S. bioeconomy toward environmental sustainability, it is critical to begin building programmatic and physical infrastructure to harness biomass, which is ultimately converted into feedstock using biotechnology applications and used in the biomanufacturing process to create everyday materials for the public.
Not All Biomass is Used for Energy, or Sustainably Produced
While biomass holds promise as a renewable energy source, not all biomass is used for energy, and not all of it is sustainable. Corn is a consistent poster child of the biomass and biofuel industry as a sustainable way to power combustion engines. Yet, the growth of corn relies heavily on the extensive use of fertilizers and pesticides, which can lead to soil erosion, water pollution, and habitat degradation. Depending on how a company conducts its Life Cycle Assessment and Carbon Intensity of its supplies, corn may not truly represent an environmentally sustainable biomass solution.
However, it is tough to beat the productivity of corn and its ability to be used for various biomass and atmospheric carbon capture applications. For example, corn stover, the byproduct stalks and leaves leftover from harvest, can be broken down into biochar for reuse in soil nutrient replenishment and is excellent for carbon sequestration from the atmosphere. Carbon sequestration is the “storage of carbon dioxide (CO2) after it is captured from industrial facilities and power plants or removed directly from the atmosphere”. One California-based company, Charm, is harvesting the leftover corn leaves, husks, and stalks and breaking them down into bio-oil which is stored deep underground in EPA-regulated wells. This bio-oil now contains sequestered carbon from corn crops and locks it away for thousands of years thus allowing a simple, and effective, way to use farm waste materials as carbon sequestration machines. This corn stover may otherwise have been burned or left to rot, releasing its carbon into the atmosphere.
“Crops can serve as a carbon sink, capturing CO2 from the atmosphere. During CO2 fermentation, some of this recycled CO2 can be harnessed for various applications, such as carbon capture and storage, where it can be compressed or stored underground. The convergence of lower input costs, improvement of ethanol production, and CO2 management showcases a sector poised to contribute to a sustainable and prosperous future.”
While corn remains the leader of the biomass pack for usage in atmospheric carbon capture, it is necessary to begin broadening the biomass portfolio into other crops, both conventional and not, that can offer similar carbon capture and biomass benefits for industrial energy and feedstock use. The introduction of more sustainable biomass inputs, like waste hulls from almond crops, winter oilseed crops, or macro/microalgae, might be the key to introducing options for industries to use for their biomanufacturing processes. To make the U.S. bioeconomy more environmentally sustainable, it will be necessary to prioritize the use of biomass that is sustainable for the creation of bio-based products. To achieve this, policymakers and industry leaders can come together to understand the physical infrastructure needed to support the processing and utilization of sustainable biomass.
Biomass in Carbon Accounting
A contentious issue in biomass utilization revolves around carbon accounting, particularly concerning the differentiation between biogenic and fossil fuel carbon. Biogenic carbon originates from recently living organisms and is part of the natural carbon cycle, while fossil fuel carbon is derived from the remains of extinct carbon-rich plants and animals that decomposed as they were compressed and heated in the ground. When burned, this fossil fuel carbon is released into the atmosphere, contributing to greenhouse gas emissions. The current carbon accounting frameworks often conflate these distinctions, leading to misconceptions and controversies surrounding biomass utilization’s carbon neutrality claims. Addressing this ambiguity is crucial for aligning policy frameworks with scientific realities and ensuring informed decision-making in biomass utilization. As microbioeconomies grow, any confusion about biogenic versus fossil fuel carbon could become another barrier to entry for burgeoning bioeconomy opportunities.
Environmental and Economic Impacts
All across rural America, local economic developers are seeing more biomass conversion projects come to their communities, which offers the chance to boost economic revenues from turning biomass into energy, fuel, or feedstock and creates a broad spectrum of jobs for the area. To capitalize on this, increased bioliteracy on how growing biomass could offer additional financial support for farmers, provide energy to heat communities, and become feedstock for the biotechnology and biomanufacturing industry is critical. The more we activate and connect parts of America that are not located in existing high-density technology hubs, the better prepared these communities will be when biomass projects look to settle in those places. For example, woody biomass was emphasized throughout the DOE 2023 Billion Ton report as an important biomass source for fuel and energy production, yet the process of getting the timber and woody biomass out of the forest and into processing facilities is slow to launch due to concerns over environmental impacts.
While environmental impacts are valid and of great concern in some ecosystems around the U.S., harvesting wood waste and timber in areas that are primed for increased forest fire risk might be a sustainable option for protecting forest ecosystems while also benefiting the community for energy and heat concerns. The USDA Wildland Fire Mitigation and Management Commission discussed the need for further research into forest biomass to understand how it can generate profit for communities with otherwise waste materials while also mitigating fire risk. One recommendation stated the need for “Increase[d] resources for programs to help private landowners dispose of woody biomass”. Although several programs assist landowners in this effort, there are still significant expenses involved. These costs may discourage landowners from conducting fuel reduction activities, leading them to either burn the material, which can harm air quality, or leave it on the land, potentially worsening wildfire severity in case of an outbreak. There’s a necessity for initiatives supporting the disposal of biomass, including wood chipping, hauling, and its utilization. These initiatives could receive support from USDA Rural Development and should explore ways to encourage landowners to sustainably harvest their woody biomass for both financial incentives and for reducing wildfire risk.
Billion Ton Report Recommendations
According to the 2023 DOE Billion-Ton Report, the U.S. used 342 million tons of biomass for energy and bio-based chemicals in 2022. The top biomass source for biofuels is corn, with the U.S. producing nearly 150 tons per year of corn that is converted to ethanol. Whereas ~140 million tons of forestry/wood and wood waste (woody biomass) are used for heat and power purposes. However, many other types of biomass exist and are used for various purposes including transportation or industrial and electrical power. Below is an abbreviated list, based on the Billion-Ton Report, of common biomass examples and some of their uses.
The recent Billion Ton report makes it clear that the U.S. has plenty of available biomass for use in the production of biofuel, heat/energy, and bio-based products, and that further utilization of biomass in these applications and in biotechnology and biomanufacturing industries could be a way forward to mitigate climate change and improve sustainability of the U.S. bioeconomy. To change the mindset of biomass as more than corn grown for biofuel, it will take a concerted effort by the federal agencies involved in funding biomass use projects, like the DOE, USDA, National Science Foundation, and the Department of Defense, to communicate to farmers that growing biomass can be profitable. It will also take a joint effort from the federal government and local governments to build pilot and commercial scale facilities to begin processing diverse biomass.
Overall, there is immense promise in connecting biomass growers, processors, and bio-powered industries. It allows the players in the U.S. bioeconomy to think critically about their waste outputs and how to harness biomass as the key to unlocking a future where all communities, be they rural or urban, benefit from our national bioeconomy. You can learn more about biomass use in biotechnology and biomanufacturing at our upcoming webinar May 1st at 10 AM ET.
Bold Goals Require Bold Funding Levels. The FY25 Requests for the U.S. Bioeconomy Fall Short
Over the past year, there has been tremendous momentum in policy for the U.S. bioeconomy – the collection of advanced industry sectors, like pharmaceuticals, biomanufacturing, and others, with biology at their core. This momentum began in part with the Bioeconomy Executive Order (EO) and the programs authorized in CHIPS and Science, and continued with the Office of Science and Technology Policy (OSTP) release of the Bold Goals for U.S. Biotechnology and Biomanufacturing (Bold Goals) report. The report highlighted ambitious goals that the Department of Energy (DOE), Department of Commerce (DOC), Human Health Services (HHS), National Science Foundation (NSF), and the Department of Agriculture (USDA) have committed to in order to further the U.S. bioeconomical enterprise.
However, these ambitious goals set by various agencies in the Bold Goals report will also require directed and appropriate funding, and this is where we have been falling short. Multiple bioeconomy-related programs were authorized through the bipartisan CHIPS & Science legislation but have yet to receive anywhere near their funding targets. Underfunding and the resulting lack of capacity has also led to a delay in the tasks under the Bioeconomy EO. In order for the bold goals outlined in the report to be realized, it will be imperative for the U.S. to properly direct and fund the many different endeavors under the U.S. bioeconomy.
Despite this need for funding for the U.S. bioeconomy, the recently-completed FY2024 (FY24) appropriations were modest for some science agencies but abysmal for others, with decreases seen across many different scientific endeavors across agencies. The DOC, and specifically the National Institute of Standards and Technology (NIST), saw massive cuts in funding base program funding, with earmarks swamping core activities in some accounts.
There remains some hope that the FY2025 (FY25) budget will alleviate some of the cuts that have been seen to science endeavors, and in turn, to programs related to the bioeconomy. But the strictures of the Fiscal Responsibility Act, which contributed to the difficult outcomes in FY24, remain in place for FY25 as well.
Bioeconomy in the FY25 Request
With this difficult context in mind, the Presidential FY25 Budget was released as well as the FY25 budgets for DOE, DOC, HHS, NSF, and USDA.
The President’s Budget makes strides toward enabling a strong bioeconomy by prioritizing synthetic biology metrology and standards within NIST and by directing OSTP to establish the Initiative Coordination Office to support the National Engineering Biology Research and Development Initiative. However, beyond these two instances, the President’s budget only offers limited progress for the bioeconomy because of mediocre funding levels.
The U.S. bioeconomy has a lot going on, with different agencies prioritizing different areas and programs depending on their jurisdiction. This makes it difficult to properly grasp all the activity that is ongoing (but we’re working on it, stay tuned!). However, we do know that the FY25 budget requests from the agencies themselves have been a mix bag for bioeconomy activities related to the Bold Goals Report. Some agencies are asking for large appropriations, while some agencies are not investing enough to support these goals:
Department of Energy supports Bold Goals Report efforts in biotech & biomanufacturing R&D to further climate change solutions
The increase in funding levels requested for FY25 for BER and MESC will enable increased biotech and biomanufacturing R&D, supporting DOE efforts to meet its proposed objectives in the Bold Goals Report.
- INCREASE: $945M for Biological and Environmental Research (BER) in FY25, a substantial increase of 5% from FY24 appropriations of $900M
- INCREASE: $113M for Manufacturing & Energy Supply Chains (MESC) in FY25, a substantial expansion from FY24 appropriations of $18 million
Department of Commerce falls short in support of biotech & biomanufacturing R&D supply chain resilience
One budgetary increase request is offset by two flat funding levels.
- INCREASE: $645M for the International Trade Administration in FY25, a 6% increase from the FY24 enacted amount
- Specifically they are asking for $103M for the Supply Chain Resiliency Program. This is important because, as seen during the COVID-19 pandemic, our supply chain currently is not resilient. Ensuring that we have the proper inputs, outputs, and materials required to continue our day-to-day endeavors will be vital in maintaining and leading a strong bioeconomy.
- FLAT: $1.5B for NIST in FY25, a 3% increase from FY24 enacted of $1.46 billion
- $37M for NIST’s Manufacturing USA program and $175M for the Manufacturing Extension Partnership, both flat from the FY24 appropriations levels. Flat funding won’t keep pace with the amount of work that needs to be done to create a resilient supply chain and programs like BioMADE and NIIMBL need to guide their industries forward.
Department of Agriculture falls short in support of biotech & biomanufacturing R&D to further food & Ag innovation
- INCREASE: $1.74B for the National Institute of Food and Agriculture, a 3.5% increase from the FY24 enacted amount
- DECREASE: $1.78B for the Agricultural Research Service program, a 3% decrease from the FY24 appropriated $1.85B
- NO FUNDING: Once again the Agriculture Advanced Research and Development Authority (AgARDA) has not been funded at all in FY25, despite being authorized at $50M a year through FY23 through the 2018 Farm Bill – this is a missed opportunity to build much needed agriculture innovation
Human Health Services falls short in support of biotech & biomanufacturing R&D to further human health
- DECREASE: $47.9B for the National Institutes of Health, a 1.4% decrease from the FY24 appropriated $48.6B
- Specifically, the Advanced Research Projects Agency for Health (ARPA-H) would receive $1.5B, maintaining funding levels seen in FY24
- DECREASE: $970M for the Biomedical Advanced Research & Development Authority (BARDA), a 4% decrease from the FY24 appropriated $1B
National Science Foundation supports Bold Goals Report efforts in biotech & biomanufacturing R&D to further cross-cutting advances
- INCREASE: $8B for Research and Related Activities within NSF, a 12% increase from the FY24 enacted amounts
- $900M for Technology, Innovation, and Partnerships (TIP), where in FY24 Congress supported the program without specifying funds for it
- $258M for the Established Program to Stimulate Competitive Research (EPSCoR), a 3% increase from the FY24 enacted amount
- INCREASE: $387M for Advanced Manufacturing across NSF, a 9% increase from FY23 amounts*
- INCREASE: $421M for Biotechnology across NSF, a 9.4% increase from FY23 amounts*
- The NSF Engines will be vital in shaping regional microbioeconomies and furthering cross-cutting advances and information on their FY25 budgets can be found here.
* FY23 amounts are listed due to FY24 appropriations not being finalized at the time that this document was created.
Overall, the DOE and NSF have asked for FY25 budgets that could potentially achieve the goals stated in the Bold Goals Report, while the DOC, USDA and HHS have unfortunately limited their budgets and it remains questionable if they will be able to achieve the goals listed with the funding levels requested. The DOC, and specifically NIST, faces one of the biggest challenges this upcoming year. NIST has to juggle tasks assigned to it from the AI EO as well as the Bioeconomy EO and the Presidential Budget. The 8% decrease in funding for NIST does not paint a promising picture for either the Bioeconomy EO and should be something that Congress rectifies when they enact their appropriation bills. Furthermore, the USDA faces cuts in funding for vital programs related to their goals and AgARDA continues to be unfunded. In order for USDA to achieve the goals listed in the Bold Goals report, it will be imperative that Congress prioritize these areas for the benefit of the U.S. bioeconomy.
Regulations, funding, and knowledge gaps: Challenges and opportunities in bringing agricultural biotechnology to market
Innovations in agriculture will play an increasingly important role in America’s quest to ensure resilient and sustainable production of food, medicine, and bioenergy products. Biotechnology, spurred by advances such as cheap sequencing, offers a realm of possibilities for novel agricultural inputs, such as more targeted pesticides that are less toxic and less likely to cause tolerance, less carbon-intensive alternatives to fertilizers, and more climate-resilient crop varieties.
However, research and development of new agricultural biotech products can be expensive and time-consuming, due to the large physical scale and long timelines of field trials. At the same time, federal funding for agriculture research has historically paled in comparison to funding for defense, energy, and human health. For example, in 2022, the NIH’s R&D budget was more than 16 times that of the USDA’s.
The Biden administration has demonstrated its recognition of the need to accelerate research and development in agricultural biotechnology, featuring it prominently in 2022’s Executive Order on Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy. Additionally, a bill to expand authorization of funding for a moonshot USDA research grant program, AgARDA (Agriculture Advanced Research and Development Authority), has broad bipartisan support. At the same time, there has been a commensurate increase in private funding.
While this multi-front surge in enthusiasm and investment is welcome, many challenges remain in translating money, ideas, and laboratory results to the field and the market, including communication between the various stakeholders in agricultural biotechnology R&D. To better understand industry priorities and potential barriers to progress, we spoke to members of the executive team of Fall Line Capital (FLC), a venture capital (VC) and private equity firm that invests in food/agriculture startups. Fall Line’s investments include new biopesticides (Greenlight, Micropep), functional microbes (Pluton, Wild Microbes), and new equipment (Guardian Agriculture, Rantizo, LUMO), in addition to managing a farmland portfolio. As lifelong farmers as well as agriculture technology (agtech) investors, Clay Mitchell and Scott Day offer a multifaceted perspective on the current landscape.
We then outline actions for government actors that can address the challenges identified in our interview, in three key areas: regulatory oversight, federal R&D funding, and bioliteracy.
Q: What can the U.S. government do to provide a supportive landscape for new agricultural biotechnology?
Fall Line Capital: I think the biggest hurdles are regulatory. If the government wants to be truly supportive and innovative, it should be working to revamp the convoluted regulatory environment. The current system wasn’t designed to handle all the new technology being developed with novel mechanisms of action, so hurdles to creating and commercializing stifle innovation even more than they did in the past.
Looking at new technology like RNA– and micropeptide-based pesticides, it’s been a difficult process to get those products registered, even though they should be embraced: compared to conventional pesticides, they have the potential to be highly specific to the target organism and minimally toxic to non-target organisms. During GreenLight’s discussions with the EPA to register their RNAi biopesticide for tackling invasive potato beetles, the EPA seemed to understand that this sort of technology is the future, but movement through the registration approval process was slow nevertheless; the application sat there for five 5 years. There were dozens of other biological pesticide product applications, and the EPA had to give every application the same level of scrutiny, even if many were obviously ineffective. There’s pressure to register more biological products as a prominent alternative to traditional chemical products, despite generally low efficacy. This clutters up the process, and the EPA was already short-staffed after extensive attrition during COVID.
A substantial amount of the innovation is coming from small companies like Greenlight that don’t have the resources (which many of the large incumbent ag companies have) to navigate the current registration programs and protocols, which are spread across multiple agencies involved in regulating biotechnology: the USDA, EPA, and FDA. There needs to be a new concierge resource beyond what the Unified Website for Biotechnology Regulation currently provides, that could direct you to the right office, the right registration process, as well as appropriate funding opportunities and legal resources.
Q: What do you think are currently the most pressing challenges in agriculture?
FLC: Pest resistance continues to be a very serious concern in agriculture, so new and effective control measures need to be continually developed for all pests: weeds, insects, disease. There are two major pests of concern for my own farm in Western Canada. First, herbicide-resistant kochia weed, which has become a huge problem in the last five years all across the world. No one’s sure how exactly it spread so quickly. Second, flea beetles are decimating cruciferous crops. RNAi-based insecticides could be very effective here, if we can achieve sufficient persistence of the insecticide and avoid impacting non-target species.
In terms of challenges to agriculture-based businesses, there’s a lack of funding right now for getting tech to market. Funding for agtech from VC firms fell last year, as it did for most forms of tech. This was following a very strong period of agtech funding for the previous two years, during which we saw over-investment in several sectors, such as alternative meat and indoor farming. At the same time, agtech companies typically have long timelines to product launch and need more funding than just one VC can provide. Right now, many companies who come to Fall Line for money are just looking for short-term “bridge funding” so they can make payroll and buy time until they can demonstrate enough progress to raise a successful “series” round with good valuation and favorable investment terms. And no one is going public right now.
Q: What are common knowledge gaps for agtech startups regarding farmers’ needs?
FLC: Agtech startups are often centered around a great idea or technology that’s looking for a problem to solve — but it’s hard for a specific technology to meet the needs of a variable problem. Farmers’ needs and priorities (e.g. pests, nutrients, etc.) are incredibly diverse, varying dramatically by crop and location, even from one field to the next on the same farm, or even within the same field. Today, it is very hard to get an accurate understanding of what is needed or desired at the farm level because there is no easy way to connect with growers on a broad scale. Farm papers have diminished in popularity just like mainstream papers, radio has diminished as well. Unless you have the email address or cell phone number of a farmer, it is hard to connect directly with them now, and most farmers don’t like doing surveys of any type anyway — and those that do aren’t that representative of the industry. I think this is why most types of polls are becoming less accurate as it is increasingly more difficult to get a representative sample of opinions.
Farmers can be hesitant to adopt new technologies, since the risk can be high. And once they’ve been burned once by a product that failed to work as advertised, they’re unlikely to be willing to trust that company, or even that type of product, in the future. For example, last year, North Dakota State University scientists coordinated a large-scale field trial where it showed in a large field trial that most new biological products aimed at improving nitrogen-fixation in non-legume crops were ineffective at increasing yield. In general, the efficacy of biologicals can vary greatly depending on the exact field conditions, making it hard to reliably achieve the advertised result. There’s a huge jump from greenhouse results to field trials, another huge jump from field trials to commercial fields. But when a product’s value is obvious, farmers actually embrace new technology very quickly: both GMO crops and GPS achieved widespread adoption in a very short period of time.
Finally, technology developers should keep in mind that problems can be solved by old or simple technology. When people think about controlled-environment farming, their minds jump to fancy things like vertical farming — but with irrigation and mulch films, you’re 90% of the way there. Simply by adding a mulch film to heat the soil, farmers can greatly extend the growing season in northern climates by a month. This approach allowed us us to substantially increase the yield from our corn fields in Wisconsin.
This conversation illustrates a clear need for change in three key areas:
Federal funding for agricultural R&D
Given the unreliability of private market funding for agricultural biotechnology R&D, which often entails long turnaround times and low margins relative to traditional tech companies, substantial federal funding through research programs such as AgARDA is vital for accelerating R&D. AgARDA, based on the ARPA Advanced Research Projects Agency model, would allow the USDA to support the development of transformative technologies for focus areas of its choosing. However, despite its popularity, AgARDA, which was first authorized in the 2018 Farm Bill for $50 million annually for FY2019-2023, only received $2m in that timeframe. The USDA requested $5m for AgARDA in FY2022 and again in FY2023; it only received $1m each year. By contrast, ARPA-H, the human health equivalent, was authorized in FY2022 and immediately received its full $1 billion authorization, followed by $1.5b in FY2023.
The USDA has published an implementation framework for AgARDA. Unfortunately, misalignment between USDA and Congress appears to be preventing AgARDA from being fully funded to its authorized levels. Members of the Congressional agriculture committees want the USDA to show that it has made progress with the $2m it has received before they allocate additional funding, namely the appointment of a dedicated director and initiation of a pilot program with calls for grant proposals. However, the USDA has deemed the $2m insufficient to support long-term staff or a formal grant program, especially since the appropriations require annual renewal. The current impasse means that no AgARDA projects have been rolled out, despite the pressing nature of the research priorities identified by the USDA.
The following steps should be taken for AgARDA to achieve its full potential:
- USDA should produce a formal report to Congress of how it utilized the $2m thus far allocated.
- Congress should ensure that the bill to expand AgARDA authorization from $50m to $100m is passed as part of the upcoming Farm Bill.
- USDA should request, and Congress should approve, funding up to its full authorization for AgARDA in its FY2026 budget. Additionally, the appropriations should be granted on a three-year basis, like ARPA-H’s appropriations, to permit greater runway.
Regulatory oversight
The U.S. regulatory system for biotechnology needs to be a) expanded, with funding for a larger agency staff to process applications quickly; b) updated, to be flexible such that it can accommodate new-to-market technologies; and c) coordinated, to streamline approval processes.
The National Security Commission on Emerging Biotechnology (NSCEB) addresses these unmet needs in its interim report. First, NSCEB is “considering options to facilitate higher staffing levels”; this should be made a priority.
Second, concerning regulatory oversight, NSCEB identified three potential paths for improvement:
- discrete changes to individual statutes to reduce redundancies and gaps in biotechnology oversight;
- a single, unified regulatory process to assess any novel risks associated with biotechnology products relative to their conventional counterparts; and
- a hybrid approach that legislatively mandates coordination while facilitating individual agency review and risk assessment.
Of these, the hybrid approach would likely provide the greatest flexibility. In contrast, discrete changes to individual statutes will likely involve slow, piecemeal changes that can easily become outdated again. While a unified regulatory process may be more streamlined, the report’s phrasing creates a sharp binary delineation between biotech and conventional that does not reflect reality. Such a delineation could engender a lot of wasted time debating biotech versus conventional classification for a given product.
Finally, to address intra- and interagency coordination, the NSCEB presented two Farm Bill amendments that deserve Congressional support: the Biotechnology Oversight Coordination Act and the Agriculture Biotechnology Coordination Act.
Bioliteracy and agricultural education
Market demand and regulations are informed by consumer perceptions, which then impact R&D decisions. For example, fear of consumer and regulatory backlash can dissuade companies from investing in new genetic engineering technology for developing new plant varieties, despite their potential to improve agricultural sustainability. Increased bioliteracy across the American public would help consumers, businesses, and policymakers alike better understand new biotechnologies and engage with the burgeoning bioeconomy. This is a need that the NSCEB has also highlighted. At the K-12 level, improvements could comprise updating science curriculums to include contemporary topics like gene editing, as well as amending civics curriculums to better explain the modern functions of regulatory agencies. In addition, agricultural education can be embedded into biology and earth science curriculums to reconnect the public at large with the realities faced by producers. Similar to computer science literacy improvements through standard setting and funding, bioliteracy can be improved through state-level education initiatives.
The Federation of American Scientists values diversity of thought and believes that a range of perspectives — informed by evidence — is essential for discourse on scientific and societal issues. Contributors allow us to foster a broader and more inclusive conversation. We encourage constructive discussion around the topics we care about.
The U.S. Bioeconomy is Not Yet Sustainable. Here’s What Needs to Change.
The U.S. Bioeconomy can be a slippery thing, but there’s no denying that leaders at the highest levels of industry and politics are paying attention to its potential to boost our economic growth and our technological edge.
Look no further than the White House’s Bioeconomy Executive Order – aimed at shaping a bioeconomy that is “safe, secure and sustainable.” While programs and reports have focused on the ‘safe’ and ‘secure’ aspects of the bioeconomy and economic indicators, environmental sustainability has not had as much momentum. But this isn’t necessarily due to a lack of interest in biobased products at the industrial level.
A novel collaboration between Ford Motor Company and Jose Cuervo® Tequila Company is one example of this growing interest. Agave by-products from tequila production will soon be used to create more sustainable bioplastics for next-generation Ford vehicles. According to the United Nations Environment Programme, 5 billion metric tons of agricultural biomass waste is produced annually. Agriculture by-products, like the waste products from tequila production, are abundant and often underutilized; therefore, finding new processes to incorporate available waste products to create something new and sustainable can help manufacturers embrace more biobased materials.
But one catch for building an environmentally sustainable national bioeconomy strategy is that not all biobased products or processes in the bioeconomy are – despite the connotations of “biobased” – inherently sustainable. Biobased products do indeed hold enormous promise for promoting economic growth while mitigating environmental challenges. And yet a strategy to ensure that environmental benefits actually get to consumers remains elusive. As the U.S. government grapples with delineating what sectors the bioeconomy does or does not contain – it must also ask: What does environmental sustainability mean in a bioeconomy? How should it be measured? Answers to these questions would support efforts to evaluate technologies and projects, prioritize investments, and ultimately improve sustainability.
Circular bioeconomy is a term commonly used to describe sustainability in the bioeconomy and combines two fundamental sustainability principles. First, it embraces the increased use of renewable resources, such as energy, chemicals, and materials, particularly those derived from plants. Second, it focuses on extending the lifecycle of these sustainable materials and products instead of discarding them.
While the U.S. grapples with defining its bioeconomy and landing on a cohesive approach to making it sustainable, the European Union (EU) and other international organizations have committed to this circular bioeconomy model (see BOX).
International definitions of the bioeconomy that include sustainability
Sustainability within the context of the European bioeconomy has been defined in many ways, including:
- Achieving economic growth, social, and environmental objectives by creating and adopting innovative solutions for the sustainable management of renewable biological resources.
- A circular, green, and inclusive approach that aims to create sustainable value from biomass and waste resources and ensure that future generations can benefit from our natural capital.
In the definitions above, economic and environmental sustainability inform each other depending on where a bioeconomy is located and what sector of the bioeconomy is being considered, such as biopharmaceutical manufacturing vs. agriculture. For example, sustainability for agriculturally-related bioeconomic products from Spain’s Andalusia region may look very different from sustainability for biopharmaceuticals from Berlin. The regionality differences will inform decisions that drive sustainability in a bioeconomy.
The United Nations Food and Agriculture Organization (FAO )is creating guidance for developing and implementing sustainable bioeconomy strategies, policies, and programs across the globe The FAO focuses on five key elements of a sustainable bioeconomy: 1) the reduction of carbon emissions, 2) restoring biodiversity, 3) eliminating toxic waste, 4) building rural economies, and 5) reducing food insecurity and malnutrition. The FAO further states that the “development of a sustainable and circular bioeconomy globally is and will be driven by three broad forces:
- Societal aspirations and good governance for sustainable development and improved health and wellbeing.
- Needs and opportunities to valorize and protect biological resources in the traditional bioeconomy core sectors.
- Scientific breakthroughs in biological, digital, and other fields of technology to expand possibilities for innovation.”
The EU is currently working towards a sustainable bioeconomy that aligns with the European Green Deal objectives, to build more diverse supply chains that are less dependent on fossil fuels and non-renewable resources. Furthermore, the EU sees the shift towards biobased products and sustainable processes as a way to achieve economic, social, and environmental goals. Establishing an aligned strategy allows for the two independent plans to work in synergy together to promote and advance EU’s goals. Which is further promoted by financial incentives that help EU member countries and municipalities to partake in this overarching strategy. Significant amounts of funding have been invested into the European bioeconomy and more member states of the EU are using tax incentives, grants, loans, and subsidies for biobased products to “provide public financial support to circular bioeconomy projects.” These efforts push the private sector to create more biobased products, but also enable a shift in manufacturing and research development processes to become more sustainable in order to capture additional financial benefits.
The EU’s intentional inclusion of sustainability as part of their bioeconomy strategy can be attributed to the general acceptance of nature as a societal and economical benefit. This sustainability-forward mindset informs how the EU seeks to use biotechnology as a tool to fix societal challenges. Furthermore, the sustainability-forward mindset has informed how natural resources are included as part of their economic evaluations. Preserving their natural resources becomes a priority for them and the EU sees the bioeconomy, and the biotechnology sitting within it, as a means to safeguarding their natural resources.
The U.S., on the other hand, has a rich history in manufacturing, and takes a more industry-forward approach to promote biomanufacturing and biotechnology as a way to create new biobased products. Any societal challenges that may be alleviated along the way come as a positive byproduct but it is not the primary focus of the U.S. bioeconomy. Inclusion of sustainability in the U.S. bioeconomy gets further stress-tested by the vastness of the U.S. natural landscape and the increasing number of natural disasters that vary from one region to another. For example, the rampant wildfires continue to destroy thousands of acres of forested land on the West Coast and the coastal habitats are lost on the East Coast due to rising ocean levels. This all leads to immense challenges in conserving and protecting these natural resources at a national level, making it hard to establish a coherent environmental sustainability strategy for the U.S. bioeconomy.
To successfully achieve environmental sustainability, the U.S. bioeconomy needs a two-pronged approach. The first approach requires incorporating sustainability at the regional level. Due to historic, place-based federal investments throughout the U.S., like the Economic Development Administration (EDA) Tech Hubs or the National Science Foundation (NSF) Regional Innovation Engines, regional bioeconomies, or microbioeconomies, are beginning to form.
Microbioeconomies utilize a region-specific biobased industries, academic strengths, and support sectors to apply and innovate on various biotechnologies that boost regional economies and mitigate region-specific environmental challenges. Microbioeconomies enable the integration of sustainability into the bioeconomy in a more approachable manner. Taking the lessons learned and major themes that arise from how these microbioeconomies are established and including sustainability in their planning, allows for a roadmap on how to integrate sustainability into the national bioeconomy strategy.
The second approach requires action at the federal level, in other words, a top-down approach to incorporating environmental sustainability into the U.S. bioeconomy. This approach would require a dedicated effort to build the necessary infrastructure and common language of what sustainability is and how it can exist within the bioeconomy. One way that the federal government can start this process is by driving the convergence of bioeconomy and sustainability programs within federal agencies. By mandating that federally-funded bioeconomy programs and activities include a component of environmental sustainability, the government can spur a new wave of innovation and encourage regional efforts to incorporate sustainability in their microbioeconomies. The federal government can leverage and fortify existing programs to carry out this approach. For example, the BioPreferred program, housed within the United States Department of Agriculture (USDA), is meant to increase the purchase of biobased products in the U.S. through mandatory purchasing requirements for federal agencies and their contractors; and a voluntary labeling initiative for biobased products. As the recent Bioeconomy Executive Order also highlighted the need to strengthen and expand the BioPreferred program, and implementation of this task can be another step forward in incorporating sustainability into the U.S. bioeconomy.
With historic levels of investments and the push to reduce emissions to tackle climate change, the time is ripe with opportunities for U.S. innovators to bring sustainability into the fold of their manufacturing and R&D processes, products, and services. The U.S can take steps in the right direction by creating financial incentive programs similar to ones implemented by EU member states, incorporating sustainability language into our federal codes, and mandating that federally funded biotechnology research have a sustainability component. These changes will be critically important to both grow a future circular bioeconomy in the U.S. that can simultaneously promote economic growth and help alleviate the impacts of climate change.
A Matter of Trust: Helping the Bioeconomy Reach Its Full Potential with Translational Governance
The promise of the bioeconomy is massive and fast-growing—offering new jobs, enhanced supply chains, novel technologies, and sustainable bioproducts valued at a projected $4 trillion over the next 16 years. Although the United States has been a global leader, advancements in the bioeconomy—whether it’s investing in specialized infrastructural hardware or building a multidisciplinary STEM workforce—are subject to public trust. In fact, public trust is the key to unlocking the full potential of the bioeconomy, and without it, the United States may fall short of long-term economic goals and even fall behind peer nations as a bioeconomy leader. Recent failures of the federal regulatory system for biotechnology threaten public trust, and recent regulations have been criticized for their lack of transparency. As a result, cross-sector efforts aim not just to reimagine the bioeconomy but to create a coordinated regulatory system for it. Burdened by decreasing public trust in the federal government, even the most coordinated regulatory systems will fail to boost the bioeconomy if they cannot instill public trust.
In response, the Biden-Harris Administration should direct a Bioeconomy Initiative Coordination Office (BICO) to establish a public engagement mechanism parallel with the biotechnology regulatory system. Citizen engagement and transparency are key to building public trust, yet current public engagement mechanisms cannot convey trust to a public skeptical of a biotechnology’s rewards in light of perceived risks. Bioeconomy coordination efforts should therefore prioritize public trust by adopting a new public-facing biotechnology evaluation program that collects data from nontraditional audiences via participatory technology assessments (pTA) and Multi-Criteria Decision Analysis (MCDA/MCDM) and provides insight that addresses limitations. In accordance with the CHIPS and Science Act, the public engagement program will provide a mechanism for a BICO to build public trust while advancing the bioeconomy.
The public engagement program will serve as a decision-making resource for the Coordinated Framework for the Regulation of Biotechnology (CFRB) and a data repository for evaluating public acceptance in the present and future bioeconomy.
Challenge and Opportunity
While policymakers have been addressing the challenge of sharing regulatory space among the three key agencies—Environmental Protection Agency (EPA), Food and Drug Administration (FDA), and USDA—transparency and public trust remain challenges for federal agencies, small to midsize developers, and even the public at large. The government plays a vital role in the regulatory process by providing guidelines that govern the interactions between the developers and consumers of biotechnology. For over 30 years, product developers have depended on strategic alliances between product developers and the government to ensure the market success of biotechnology. The marketplace and regulatory oversight are tightly linked, and their impacts on public confidence in the bioeconomy cannot be separated.
When it comes to a consumer’s purchase of a biotechnology product, the pivotal factor is often not price but trust. In 2016, the National Academy of Sciences, Engineering, and Medicine released recommendations on aligning public values with gene drive research. The report revealed that public engagement that promotes a “bi-directional exchange of information and perspectives” can increase public trust. Moreover, a 2022 report on Gene Drives in Agriculture highlights the importance of considering public perception and acceptance in risk-based decision-making.
The CHIPS and Science Act provides an opportunity to address transparency and public trust within the federal regulatory system for biotechnology by directing the Office of Science and Technology Policy (OSTP) to establish a Coordination Office for the National Engineering Biology Research and Development Initiative. The coordination office (i.e., BICO) will serve as a point of contact for cross-sector engagement and create a junction for the exchange of technical and programmatic information. Additionally, the office will conduct public outreach and produce recommendations for strengthening the bioeconomy.
This policy window presents a novel opportunity to create a regulatory system for the bioeconomy that also encompasses the voice of the general public. History of requests for information, public hearings, and cross-sector partnerships demonstrates the public’s capacity—or at least specific subsets of experts therein—to fill gaps, oversights, and ambiguities within biotechnology regulations.
While expert opinion is essential for developing regulation, so too are the opinions of the general public. Historically, discussions about values, sentiments, and opinions on biotechnology have been dominated by technical experts (for example, through debates on product vs. process, genetically engineered vs. genetically modified organisms, and perceived safety). Biotechnology discourse has primarily been restricted to these traditional, technical audiences, and as a result, public calls to address concerns about biotechnology are drowned out by expert opinions. We need a mechanism for public engagement that prioritizes collecting data from nontraditional audiences. This will ensure sustainable and responsible advancements in the bioeconomy.
If we want to establish a bioeconomy that increases national competitiveness, then we need to increase the participation of nontraditional audiences. Although some public concerns are unlikely to be allayed through policy change (e.g., addressing calls for a ban on genetically engineered or modified products), a public engagement program could identify the underlying issue(s) for these concerns. This would enable the adoption of comprehensive strategies that increase public trust, further sustaining the bioeconomy.
Research shows that public comment and notice periods are less likely to hear from nontraditional audiences—that is, members of underserved communities, workers, smaller market entities, and new firms. Despite the statutory and capacity-based obstacles federal agencies face in increasing public participation, the Executive Office of the President seeks to broaden public participation and community engagement in the federal regulatory process. Public engagement programs provide a platform to interact with interested parties that represent a wide range of perspectives. Thus, information gathered from public engagement could inform future proposed updates to the CFRB and the regulatory pathways for new products. In this way, the public opinions and sentiments can be incorporated into a translational governance framework to bring translational value to the bioeconomy. Since increasing public trust is complementary to advancing the bioeconomy, there is a translational value in strategically integrating a collective perception of risk and safety into future biotechnology regulation. In this case, translational governance allows for regulation that is informed by science and is responsive to the values of citizens, effectively introducing a policy lever that improves the adoption of, and investment in, the U.S. bioeconomy.
The future of biotechnology regulation is an emerging innovative ecosystem. The path to accomplishing economic goals within this ecosystem requires a new public-facing engagement mechanism framework that satisfies congressional directives and advances the bioeconomy. This framework provides a BICO with the scaffolding necessary to create an infrastructure that invites public input and community reflection and the potential to decrease the number of biotechnologies that fail to reach the market. The proposed public engagement mechanism will work alongside the current regulatory system for biotechnology to enhance public trust, improve interagency coordination, and strengthen the bioeconomy.
Plan of Action
To reach our national bioeconomic policy goals, the BICO should use a public engagement program to solicit perspectives and develop an understanding of non-economic values, such as deeply held beliefs about the relationship between humans and the environment or personal or cultural perspectives related to specific biotechnologies. The BICO should devote $10 million over five years to public engagement programs and advisory board activities that (a) report to the BICO but are carried out through external partnerships; (b) provide meaningful social data for biotechnology regulation while running parallel to the CFRB regulatory system; and (c) produce a repository of public acceptance data for horizon scanning. These programs will inform regulatory decision-making, increase public trust, and achieve the congressional directives outlined in Sec. 10402 of the CHIPS & Science Act.
Recommendation 1. Establish a Bioeconomy Initiative Coordination Office (BICO) as a home office for interagency coordination.
The BICO should be housed within the Office of Science and Technology Policy (OSTP). The creation of a BICO is in alignment with the mandates of Executive Order (EO) 14081, Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure Bioeconomy, and the statutory authority granted to the OSTP through the CHIPS and Science Act.
Congress should allocate $2 million annually for five years to the BICO to carry out a public engagement program and advisory board activities in coordination with the EPA, FDA, and USDA.
The public engagement program would be housed within the BICO as a public-facing data-generating mechanism that parallels the current federal regulatory system for biotechnology.
The bioeconomy cuts across sectors (e.g., agriculture, health, materials, energy) and actively creates new connections and opportunities for national competitiveness. A thriving bioeconomy must ensure regulatory policy coherence and alignment among these sectors, and the BICO should be able to comprehensively integrate information from multiple sectors into a strategy that increases public awareness and acceptance of bioeconomy-related products and services. Public engagement should be used to build a data ecosystem of values related to biotechnologies that advance the bioeconomy.
Recommendation 2. Establish a process for public engagement and produce a large repository of public acceptance data.
Public acceptance data will be collected alongside the “biological data ecosystem,” as referenced by the Biden Administration in EO 14081, that advances innovation in the bioeconomy. To provide an expert element to the public engagement process, an advisory board should be involved in translating public acceptance data (opinions on how biotechnologies align with values) into policy suggestions and recommendations for regulatory agencies. The advisory board should be a formal entity recognizable under the Federal Advisory Committee Act (FACA) and the Freedom of Information Act (FOIA). It should be diverse but not so large that it becomes inefficient in fulfilling its mandate. Striking a balance between compositional diversity and operational efficiency is critical to ensuring the board provides valuable insights and recommendations to the BICO. The advisory board should consist of up to 25 members, reflect NSF data on diversity and STEM, and include a diverse range of citizens, from everyday consumers (such as parents, young adults, and patients from different ethnic backgrounds) to specialists in various disciplines (such as biologists, philosophers, hair stylists, sanitation workers, social workers, and dietitians). To promote transparency and increase public trust, data will be subject to FACA and the FOIA regulations, and advisory board meetings must be accessible to the public and their details must be published in the Federal Register. Additionally, management and applications of any data collected should employ CARE (Collective Benefit, Authority to Control, Responsibility, Ethics) Principles for Indigenous Data Governance, which complement FAIR (Findable, Accessible, Interoperable and Reusable) Principles. Adopting CARE brings a people and purpose orientation to data governance and is rooted in Indigenous Peoples’ sovereignty.
The BICO can look to the National Science and Technology Council’s published requests for information (RFI) and public meetings as a model for public engagement. BICO should work with an inclusive network of external partners to design workshops for collecting public acceptance data. Using participatory technology assessments (pTA) methods, the BICO will fund public engagement activities such as open-framing focus groups, workshops, and forums that prioritize input from nontraditional public audiences. The BICO office should use pre-submission data, past technologies, near-term biotechnologies, and, where helpful, imaginative scenarios to produce case studies to engage with these audiences. Public engagement should be hosted by external grantees who maintain a wide-ranging network of interdisciplinary specialists and interested citizens to facilitate activities.
Qualitative and quantitative data will be used to reveal themes, public values, and rationales, which will aid product developers and others in the bioeconomy as they decide on new directions and potential products. This process will also serve as the primary data source for the public acceptance data repository. Evolving risk pathways are a considerable concern for any regulatory system, especially one tasked with regulating biotechnologies. How risks are managed is subject to many factors (history, knowledge, experience product) and has a lasting impact on public trust. Advancing the bioeconomy requires a transparent decision-making process that integrates public input and allows society to redefine risks and safety as a collective. Public acceptance data should inform an understanding of values, risks, and safety and improve horizon-scanning capabilities.
Conclusion
As the use of biotechnology continues to expand, policymakers must remain adaptive in their regulatory approach to ensure that public trust is acquired and maintained. Recent federal action to boost the bioeconomy provides an opportunity for policymakers to expand public engagement and improve public acceptance of biotechnology. By centralizing coordination and integrating public input, policymakers can create a responsive regulatory convention that advances the bioeconomy while also building public trust. To achieve this, the public engagement program will combine elements of community-based participatory research, value-based assessments, pTA, and CARE Principles for Indigenous Data Governance. This approach will create a translational mechanism that improves interagency coordination and builds public trust. As the government works to create a regulatory framework for the bioeconomy, the need for public participation will only increase. By leveraging the expertise and perspectives of a diverse range of interested parties, policymakers can ensure that the regulatory framework is aligned with public values and concerns while promoting innovation and progress in the U.S. bioeconomy.
Translational governance focuses on expediting the implementation of regulations to safeguard human health and the environment while simultaneously encouraging innovation. This approach involves integrating non-economic values into decision-making processes to enhance scientific and statutory criteria of risk and safety by considering public perceptions of risk and safety. Essentially, it is the policy and regulatory equivalent of translational research, which strives to bring healthcare discoveries to market swiftly and safely.
The Office of Science and Technology Policy (OSTP) should use translational governance through public engagement as a backbone of the National Engineering Biology Research and Development Initiative. Following the designation of an interagency committee by the OSTP—and once established under the scope of direction outlined in Sec. 10403 of the CHIPS and Science Act—the Initiative Coordination Office should use a public engagement program to support the following National Engineering Biology Research and Development Initiative congressional directives (Sec. 10402):
- 1) Supporting social and behavioral sciences and economics research that advances the field of engineering biology and contributes to the development and public understanding of new products, processes, and technologies.
- 2) Improving the understanding of engineering biology of the scientific and lay public and supporting greater evidence-based public discourse about its benefits and risks.
- 3) Supporting research relating to the risks and benefits of engineering biology, including under subsection d: Ensuring, through the agencies and departments that participate in the Initiative, that public input and outreach are integrated into the Initiative by the convening of regular and ongoing public discussions through mechanisms such as workshops, consensus conferences, and educational events, as appropriate].
- 4) Expanding the number of researchers, educators, and students and a retooled workforce with engineering biology training, including from traditionally underrepresented and underserved populations.
- 5) Accelerating the translation and commercialization of engineering biology and biomanufacturing research and development by the private sector.
- 6) Improving the interagency planning and coordination of federal government activities related to engineering biology.
In 1986, the newly issued regulatory system for biotechnology products faced significant statutory challenges in establishing the jurisdiction of the three key regulatory agencies (EPA, FDA, and USDA). In those early days, agency coordination allowed for the successful regulation of products that had shared jurisdiction. For example, one agency would regulate the plant in the field (USDA), and another would regulate the feed or food produced by the plant (FDA/DHHS). However, as the biotechnology product landscape has advanced, so has the complexity of agency coordination. For example, at the time of their commercialization, plants that were modified to exhibit pesticidal traits, specific microbial products, and certain genetically modified organisms cut across product use-specific regulations that were organized according to agency (i.e., field plants, food, pesticides). In response, the three key agencies have traditionally implemented their own rules and regulations (e.g., EPA’s Generally Recognized as Safe, USDA’s Am I Regulated?, USDA SECURE Rule). While such policy action is under their statutory authorities, it has resulted in policy resistance, reinforcing the confusion and lack of transparency within the regulatory process.
Since its formal debut on June 26, 1986, the CFRB has undergone two major updates, in 1992 and 2017. Additionally, the CFRB has been subject to multiple memorandums of understanding as well as two executive orders across two consecutive administrations (Trump and Biden). With the arrival of The CHIPS and Science Act (2022) and Executive Order 14081, the CFRB will likely undertake one of its most extensive updates—modernization for the bioeconomy.
According to the EPA, when the CFRB was issued in 1986, the expectation was that the framework would respond to the experiences of the industry and the agencies and that modifications would be accomplished through administrative or legislative actions. Moreover, upon their release of the 2017 updates to the CFRB, the Obama Administration described the CFRB as a “flexible regulatory structure that provides appropriate oversight for all products of modern biotechnology.” With this understanding, the CFRB is designed to be iterative and responsive to change. However, as this memo and other reports demonstrate, not all products of modern biotechnology are subject to appropriate oversight. The opportunity loss between addressing regulatory concerns and acquiring the regulations necessary to capitalize on the evolving biotechnology landscape fully presents a costly delay. The CFRB is falling behind biotechnology in a manner that hampers the bioeconomy—and likely—the future economy.