
An Innovation Agenda for Addiction: Breakthrough Medicines That Scale
The federal government should expand the FDA’s priority review voucher program (PRV) and provide market exclusivity advantages to encourage the development of medications for addiction.
Taken together, substance use disorders (alcohol, cigarettes, and other drugs) cause more deaths in the U.S. every year than cancer or heart disease and cause devastating downstream social harms. Despite this, only 3% of eligible patients received substance use disorder (SUD) medication, a result of low uptake and efficacy of existing medications and a lack of options for patients addicted to stimulants. This is due to a near-total absence of pharmaceutical research and development activity. To make population level impact to reduce harms from opioids, methamphetamine, cocaine, alcohol, and cigarettes, we must address the broken market dynamics in addiction medicine.
The PRV program should be expanded to cover opioid use disorder, alcohol use disorder, stimulant use disorder, and smoking. In addition, drugs that are approved for these SUD indications should have extended exclusivity and sponsors that develop these medications should receive vouchers to extend exclusivity for other medications.
Challenge and Opportunity
Addiction policy efforts on both the left and the right have struggled. Despite substantial progress reducing smoking, 29 million Americans still smoke cigarettes and feel unable to quit and 480,000 Americans die each year from smoking. While overdose deaths from opioids, cocaine, and methamphetamine have fallen slightly from their peak in 2022, they are still near record highs, three times higher than 20 years ago. Alcohol deaths per capita have doubled since 1999.
Roughly 60% of all crimes and 65% of violent crimes are related to drugs or alcohol; and the opioid crisis alone costs the United States $1.5 trillion a year. Progress in reducing addiction is held back because people with a substance use disorder take medication. This low uptake has multiple causes: in opiate use disorder, uptake is persistently low despite recent relaxations of prescription rules, with patients reporting a variety of reasons for refusal; treatments for alcohol use disorder have modest effects; and there are no approved treatments for stimulant use disorder. Only three percent take SUD medications, as shown in figure 1 below [link to image]. In brief, only 2% of those suffering alcohol use disorder, 13% of those with opiate use disorder, 2% of smokers, and approximately 0% of illicit stimulant users are receiving medication, giving a weighted average of about 3%.
There has been rapid innovation in the illicit market as synthetic opioids and expanded meth production have lowered price and increased strength and availability. Meanwhile, there has been virtually no innovation in medicines to prevent and treat addiction. The last significant FDA approval for opioid use disorder was buprenorphine in 2002; progress since then has been minimal, with new formulations or dosing of old medications. For alcohol use disorder, the most recent was acamprosate in 2004 (and it is rarely prescribed due to limited efficacy and three times a day dosing).
None of the ten largest pharmaceutical companies have active addiction medicine programs or drug candidates, and the pharmaceutical industry as a whole has only pursued minimal drug development. According to the trade association BIO, “Venture investment into companies with novel addiction drug programs over the last 10 years is estimated at $130M, 270 times less than oncology.”
There are promising addiction drug candidates being studied by academics but without industry support they will never become medicines. If pharmaceutical companies spent just 10% of what they spend on obesity therapies, we might quickly make progress.
For example, GLP-1 medicines like Ozempic and Mounjaro have strong anti-addictive effects across substances. Randomized trials and real-world patient health record studies show dramatic drops in consumption of drugs and alcohol for patients taking a GLP-1. Many addiction scientists now consider these compounds to be the biggest breakthrough in decades. However, Novo Nordisk and Eli Lilly, who own the drugs currently in the market, do not plan to run phase 3 addiction trials on them, due to fear of adverse events in substance use disorder populations. The result is that a huge medical opportunity is stuck in limbo indefinitely. Fortunately, Lilly has recently signaled that they will run trials on related compounds, but remain years from approval.
Conversations with industry leaders make clear that large pharmas avoid SUD indications for several reasons. First, their upside appears limited, since current SUD medications have modest sales. Second, like other psychiatric disorders, the problem is challenging given the range and complexity of neurological targets and the logistical challenges of recruiting people with substance use disorder as participants. Finally, companies face downside reputational and regulatory risk if participants, who face high baseline rates of death from overdose regardless, were to die in trials. In the case of Ozempic and Mounjaro, sponsors face an obstacle some have termed the “problem of new uses” – clinical trials of an already lucrative drug for a new indication carry downside risk if new side effects or adverse events are reported.
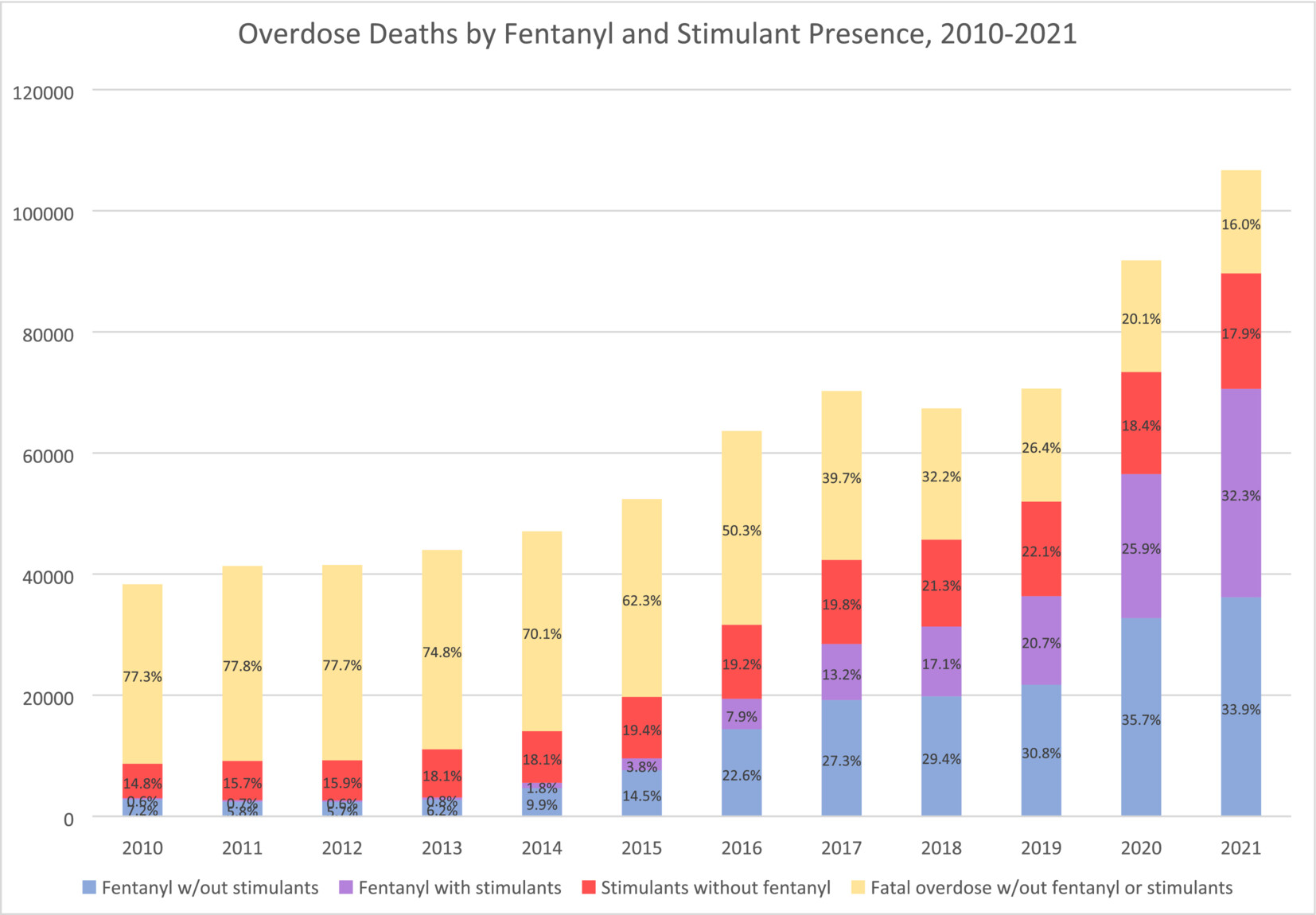
Image from Charting the fourth wave, based on CDC data
Plan of Action
Market Shaping Interventions
Recommendation 1. Expand the FDA priority review voucher (PRV) program to include addiction medicine indications.
The FDA priority review voucher (PRV) program incentivizes development of drugs for rare pediatric and infectious diseases by rewarding companies who get drugs approved with a transferable voucher that accelerates FDA approval. These vouchers are currently selling for an average of $100M. The PRV program doesn’t cost the government any money but it makes drug development in the designated categories much more lucrative. The PRV program has proven very successful, leading to a surge in approvals of medications.
As a neglected market with urgent unmet medical and public health needs, and which also promises to benefit the broader public by reducing the negative externalities of addiction, addiction medicine is a perfect fit for the PRV program. Doing so could transform the broken market dynamics of the field. The advantage of the PRV program is that it does not require substantial new congressional appropriations, though it will require Congress giving the FDA authority to expand the PRV program, as it has done previously to add other disease areas.
Recommendation 2. Extend exclusivity for addiction medicines and incentivize pursuit of new indications
Market exclusivity is a primary driver of pharmaceutical industry revenue. Extending exclusivities would have a very large effect on industry behavior and is needed to create sufficient incentives. The duration of exclusivity for alcohol use disorder, opioid use disorder, stimulant use disorder, and smoking cessation indications should be extended along with other incentives.
- Addiction medicine indications should receive an additional two years of exclusivity for biologics and three years for small molecules.
- Companies that achieve an indication for a substance use disorder for a medication that represents a significant advance would receive an exclusivity voucher that can be transferred to another medication. For 2nd, 3rd, and 4th SUD indications with the same compound, companies would be granted a shorter duration exclusivity voucher. Durations would be tiered, as described in this proposal from Duke, to balance public interest and reward levels.
- FDA should provide increased consideration for addiction medicines for breakthrough, fast track, and priority review designations, as well as accelerate meeting schedules, all of which substantially reduce development expenses.
For precedent, there are already a number of FDA programs that extend medication exclusivity, including ‘orphan drug exclusivity’ and the qualified infectious disease product (QIDP) program. Like rare diseases and antibiotics, addiction is a market that requires incentives to function effectively. In addition, successful treatments, given the negative externalities of addiction, have public benefit beyond the direct medical impact, and deserve additional public incentives.
Recommendation 3. Modernize FDA Standards of Efficacy for Substance Use Disorder Trials
A significant barrier to pharmaceutical innovation in SUDs is outdated or unpredictable efficacy standards sometimes set by the FDA for clinical trials. Efficacy expectations for substance use disorder indications are often rooted in abstinence-only and other binary measure orientations that the scientific and medical community has moved past when evaluating substance use disorder harms.
This article in the American Journal of Drug and Alcohol Abuse demonstrates that binary outcome measures like ‘number of heavy drinking days’ (NHDD) can underestimate the efficacy of treatments. This recent report from NIAAA on alcohol trial endpoints recommends a shift away from abstinence-based endpoints and towards more meaningful consumption-based endpoints. This approach should be adopted by the FDA for all SUD treatments, not just alcohol.
There are some indications that the FDA has begun modernizing their approach. This recent paper from NIH and FDA on smoking cessation therapies provides updated guidance that moves in the right direction.
More broadly, the FDA should work to adopt endpoints and standards of efficacy that mirror standards in other disease areas. This shift is best achieved through new guidance or statements issued by the FDA, which would offer positive assurance to pharmaceutical companies that they have achievable paths to approval. Predictability throughout the medication development life cycle is absolutely essential for companies considering investment.
Congress should include statements in upcoming appropriations and authorizations that state:
- The FDA should adopt non-binary standards of efficacy for addiction treatments that are aligned with standards for other common disorders and the FDA shall, within 12 months, report on the standards employed for substance use disorder relative to other prevalent chronic conditions and report steps to eliminate disparities in evidentiary standards and issue new guidance on the subject.
- The FDA should publish clear guidance on endpoints across SUDs to support planning among pharmaceutical companies considering work in this field.
Conclusion
Sustained focus and investment in diabetes and heart disease treatments has enabled medical breakthroughs. Addiction medicine, by contrast, has been largely stagnant for decades. Stimulating private-sector interest in addiction medicine through regulatory and exclusivity incentives, as well as modernized efficacy standards, is essential for disrupting the status quo. Breakthroughs in addiction medicine could save hundreds of thousands of lives in the US and provide long-term relief for one of our most intractable social problems. Given the negative externalities of addiction, this would also have enormous benefits for society at large, reducing crime and intergenerational trauma and saving money on social services and law enforcement.
This action-ready policy memo is part of Day One 2025 — our effort to bring forward bold policy ideas, grounded in science and evidence, that can tackle the country’s biggest challenges and bring us closer to the prosperous, equitable and safe future that we all hope for whoever takes office in 2025 and beyond.
PLEASE NOTE (February 2025): Since publication several government websites have been taken offline. We apologize for any broken links to once accessible public data.
Per author conversations with industry leaders, private sector interest in SUD medication development is limited for the following reasons:
- The upside of pursuing SUD indications appears limited, since current SUD medications, which are generally targeted for specific substances, have modest sales.
- Even with preliminary evidence that GLP-1 drugs may be efficacious for some SUD indications (e.g, alcohol, opiates, and tobacco), companies are reluctant to pursue label expansion for SUD. As described previously, with already lucrative drugs, companies face a downside risk (termed the “problem of new uses”) from running large clinical trials, and possibly uncovering new side effects or incurring random adverse events which could harm reputation and existing markets.
- In the specific case of SUD, this downside risk might be especially large, since people with substance use disorder have high baseline rates of overdose and death.
Moreover, there is an argument that a treatment for SUD is a public good, to the degree that it ameliorates the negative externalities of addiction – increasing the case for more public-sector incentives for SUD treatment. The end result is that medical treatments for SUD are stuck in an indefinite limbo, with private-sector interest in SUD, as documented previously, being very low.
The current lack of effective and widely used SUD medications is disheartening, but this is in the context of private sector disinterest and scant funding. Even modest successes in SUD treatment have the potential to kickstart an innovation loop, akin to the rush of biotech companies hastening to enter the obesity treatment field. Prior to the success of the GLP-1 drugs, obesity treatment had been moribund, and viewed pessimistically in light of drugs that had limited efficacy or had been withdrawn for side effects like suicidality or cardiovascular issues.
An SUD success like GLP-1 for obesity has the potential to kindle a similar rush of interest; the challenge is the initiation of that cascade. Given the very low levels of investment in SUD treatments, there is potential low-hanging fruit that, given sufficient funding, could be trialed and deployed.
There has been rapid innovation in the field of addiction, but it’s been happening on the wrong side: addiction-inducing technologies are becoming more powerful, while SUD treatments have largely stagnated. This innovation is most evident in synthetic opioids and methamphetamine.
Compared to heroin, fentanyl is about 25x stronger (on a per-weight basis), and hence, much easier to smuggle. As the Commission on Combating Synthetic Opioid Trafficking put it:
Single-digit metric tonnage of pure fentanyl is not a large amount and could easily fit into a shipping container or a truck trailer, which seriously challenges interdiction…Perhaps as much as 5 MT [metric tons] of pure fentanyl would be needed to satisfy the entire annual U.S. consumption for illegally supplied opioids.
Moreover, as a recent Scientific American article documented, innovations in fentanyl production, including the use of safer precursors and methods that don’t require sophisticated equipment, mean that fentanyl production is now decentralized, and resistant to attempts by law enforcement to shut it down.
As fentanyl has come to dominate the opioid supply over the past 10 years, overdose deaths have risen dramatically. New synthetic opioids and non-opioids like xylazine are also becoming common.
At the same time, due to advances in production techniques in Mexico, methamphetamine production has skyrocketed in recent decades while purity has improved. Worst of all, unlike heroin, fentanyl is easily combined with meth and cocaine in pills and powder.
The DEA has highlighted the presence of “super labs” in Mexico capable of producing hundreds of pounds of meth per batch.
Together, these three innovations (fentanyl, cheap meth, and new combinations) have led to a 400% increase in overdose deaths in the past 20 years. Without equally powerful innovations to reduce addiction rates, we will never make long-term and sustainable progress.
At a time when universities are already facing intense pressure to re-envision their role in the S&T ecosystem, we encourage NSF to ensure that the ambitious research acceleration remains compatible with their expertise.
FAS CEO Daniel Correa recently spoke with Adam Marblestone and Sam Rodriques, former FAS fellows who developed the idea for FROs and advocated for their use in a 2020 policy memo.
When the U.S. government funds the establishment of a platform for testing hundreds of behavioral interventions on a large diverse population, we will start to better understand the interventions that will have an efficient and lasting impact on health behavior.
Integrating AI tools into healthcare has an immense amount of potential to improve patient outcomes, streamline clinical workflows, and reduce errors and bias.