An Overdue Fix for Pulse Oximeters
The invention of pulse oximeters in the 1980s reshaped healthcare. While tracking blood oxygen content (commonly recognized as the “fifth vital sign”) once required a painful blood draw and time-delayed analysis, pulse oximeters deliver nearly instantaneous data by simply sending a pulse of light through the skin. Today, pulse oximeters today are ubiquitous: built into smartwatches, purchased at pharmacies for home health monitoring, and used by clinicians to inform treatment of everything from asthma to heart failure to COVID-19. Emerging algorithms are even incorporating pulse ox data to predict future illness.
There is a huge caveat. Pulse oximeters are medically transformative, but racially biased. The devices work less accurately on dark-skinned populations because melanin, the chemical which gives skin pigment, interferes with light-based pulse ox measurements. This means that dark-skinned individuals can exhibit normal pulse ox readings, but be suffering from hypoxemia or other critical conditions.
But because regulations to this day do not require diversity in medical device evaluation, many pulse ox manufacturers don’t test their devices on diverse populations. And because the Food and Drug Administration (FDA) has created streamlined pathways to approve new medical devices based on technology that is “substantially similar” to already-approved technology, the racial bias embedded in ‘80s-era pulse ox technology continues to pervade pulse oximeters on the market today.
COVID-19 illustrated, in devastating fashion, the consequences of this problem. Embedded bias in pulse oximeters demonstrably worsened outcomes for patient populations already disproportionately impacted by COVID-19. Studies show, for instance, that Black COVID-19 patients have been 29% less likely to receive supplemental oxygen on time and three times as likely to suffer occult hypoxemia during the pandemic.
Similar inequities persist across the health-innovation ecosystem. Women suffer from lack of sex-aware prescription drug dosages. Minorities increasingly suffer from biased health risk-assessment algorithms. Children and those with varying body types suffer from medical equipment not built for their physical characteristics. Across the board, inequities create greater risks of morbidity and mortality and contribute to ballooning national healthcare costs.
This need not be the status quo. If health stakeholders—including patient advocates, medtech companies, clinicians, researchers, and policymakers—collectively commit to systematic evaluation and remediation of bias in health technology, change is possible.
An excellent example is eGFR algorithms. These algorithms, used to assess kidney functionality, previously used faulty “correction factors” to account for patient race. But this correction did not actually correlate with biological realities—and instead of treating patients more effectively, it increased disparities in care. Motivated by the data, advocacy and industry organizations issued broad recommendations to avoid using the eGFR calculation. Hospitals and medical systems listened, dropping eGFR from practice, and the National Institutes of Health (NIH) is now committing funding to investigate alternative calculations.
We as a society must continue to root out bias in health technology, from development to testing to deployment.
When we develop new medical tools, we should consider all the populations who could ultimately need them.
When we test tools, we should rigorously evaluate outcomes across subgroup populations, looking for groups that might fare better or worse from its use in care.
And when we deploy technologies, we need to be ready to track the outcomes of their use at scale.
Engineers, researchers, and clinicians can support these goals by designing medical devices with equity in mind. The UK just launched its evidence-gathering process on equity in medical devices, looking into the impacts of bias and ways to build more equitable solutions. The FDA’s meeting reviewing the evidence on pulse oximetry is a start to auditing technologies for their performance on different populations.
Advocacy organizations can support these goals by providing input to ongoing policy processes. The Federation of American Scientists (FAS), alongside the University of Maryland Medical System, submitted a public comment to the FDA to call for regulations that will encourage the development of low-bias and bias-free tools. FAS is also convening a Forum on Bias in Pulse Oximetry to examine the consequences of bias, build an evidence base for bias-free pulse oximetry, and look ahead to approaches to build more equitable devices.
“Do no harm”, a central oath in medicine, is becoming exceedingly difficult in our technological age. Yet, with an evidence-based approach that ensures technologies equitably serve all groups in a population and works to correct them when they do not, we can come closer to achieving this age-old goal.
Pandemic Readiness Requires Bold Federal Financing for Vaccines
Summary
Most people will experience a severe pandemic within their lifetime, and the world remains dangerously unprepared. In fact, scientists predict a nearly 50% chance––the same probability as flipping heads or tails on a coin––that we will endure another COVID-19-level pandemic within the next 25 years. Shifting America’s pandemic response capability from reactive to proactive is, therefore, urgent. Failure to do so risks the country’s welfare.
Getting ahead of the next pandemic is impossible without government financing. Vaccine production is costly, and these expenses will hinder industries from preemptively developing the tools needed to halt disease transmission. For example, the total expected revenues over a 20-year vaccine patent lifecycle would cover just half of the upfront research and development (R&D) costs.
However, research suggests that a portfolio-based approach to vaccine development — especially now with new, broadly applicable mRNA technology — dramatically increases the returns on investment while also guarding against an estimated 31 of the next 45 epidemic outbreaks. With lessons learned from Operation Warp Speed, Congress can deploy this approach by (i) authorizing and appropriating $10 billion to the Biomedical Advanced Research and Development Authority (BARDA) (ii) developing a vaccine portfolio for 10 emerging infectious diseases (EIDs), and (iii) a White House Office of Science and Technology Policy (OSTP)-led interagency effort focused on scaling up production of priority vaccines.
Challenge & Opportunity
The COVID-19 pandemic continues to wreak havoc across the world, with an ongoing total cost of $16 trillion and more than 6 million dead. Three conditions increase the likelihood that we will experience another pandemic that is just as disastrous:
- New outbreaks of infectious diseases––like ––are emerging due to population growth, increased zoonotic transmission from animals, habitat loss, climate change, and more. Over 1.6 million yet-to-be-discovered, human-infecting viral species are thought to exist in mammals and birds.
- More laboratories are handling dangerous pathogens around the world, which increases the likelihood of an accidental contagion release.
- It is easier than ever to purchase biotechnologies once reserved only for scientists. Consequently, malign actors now have more resources to develop a human-engineered bioweapon.
The United States and the rest of the world are still woefully unprepared for future pandemic or epidemic threats. The lack of progress is largely due to little to no vaccine development for these six EIDs, all of which have pandemic potential:
- Middle East respiratory syndrome coronavirus (MERS-CoV)
- Lassa fever virus
- Nipah virus
- Rift Valley fever virus
- Chikungunya virus
- Ebola virus
Failure to produce and supply vaccines doses to Americans could undermine the U.S. government’s response to a vaccine crisis. This is illustrated in the recent monkeypox response. The federal government invested in a new monkeypox vaccine with a significantly longer shelf life. While focused on this effort, it failed to replace its existing vaccine stockpile as it expired, leaving the American population woefully unprepared during the recent monkeypox outbreak.
An immediate national strategy is needed to course correct, the beginnings of which are articulated in the recent plan for American Pandemic Preparedness: Transforming our Capabilities. These overarching concerns were also echoed in a bipartisan letter from the Senate Health, Education, Labor, and Pensions and Armed Services Committees, urging the Biden Administration to re-establish a “2.0” version of Operation Warp Speed (OWS)––the government’s prior effort to accelerate COVID-19 vaccine production.
The President’s recent FY23 Budget advocates for a historic pandemic preparedness investment. The plan allocates nearly $40 billion to the Department of Health and Human Services Assistant Secretary for Preparedness and Response to “invest in advanced development and manufacturing of countermeasures for high priority threats and viral families, including vaccines, therapeutics, diagnostics, and personal protective equipment.” BARDA also declared the need to prepare prototype vaccines for virus families with pandemic potential and has included such investments in its most recent strategic plan. And, the recent calls for increased “piloting and prototyping efforts in biotechnology and biomanufacturing to accelerate the translation of basic research results into practice.”
Robust federal investment in America’s vaccine industry is especially needed since––as demonstrated by COVID-19––industries garner minimal profit from vaccine development before or during a widespread outbreak. A recent study predicted that in the unlikely scenario where 10 million vaccines are manufactured during a crisis response, pharmaceutical companies can expect to recoup only half of the upfront R&D costs. The same research states that “new drug development has become slower, more expensive, and less likely to succeed” because:
- The probability of developing a successful vaccine candidate is low.
- A lengthy investment time (i.e., a long investment horizon) is required before selling for profit is possible.
- Clinical trials are very expensive.
- To justify and overcome all costs, a high financial return is needed (i.e., there is a high cost of capital).
With clinical costs accounting for 96% of total investment, companies have a weak financial justification for investing in risky vaccine research.
To minimize these uncertainties and improve investment returns for vaccine and therapeutic production, the federal government should embrace two key lessons from OWS:
- Guaranteed government demand enables the pursuit of innovative, speedy, and effective vaccine R&D. OWS selected companies pursuing different scientific methods to develop a vaccine, each of which possessed breakthrough potential. Moderna and Pfizer/BioNTech utilized mRNA, AstraZeneca and Janssen worked with replication-defective live vectors, and Novavax and Sanofi/GSK utilized a recombinant protein. Merck is working on a live attenuated virus that may be given orally. By frequently evaluating vaccine candidates, scientists ensured that only the most promising contenders continued to subsequent regulatory phases. This workflow dramatically expedited vaccine development. Relatedly, companies were able to invest in large-scale vaccine manufacturing during clinical trials thanks to government financial support. They not only received guaranteed investment installments, but also advanced commitments to purchase vaccines. This significantly decreased the financial risk and saved tremendous amounts of time and resources.
- Public-private partnerships utilize incentives and rewards to foster highly effective and dynamic teams. OWS created a “unique distribution of responsibilities … based upon core competencies rather than on political or financial considerations.” The interests of eight pharmaceutical companies were aligned based on the potential to receive an upfront commitment from the federal government to bulk purchase vaccines. Such approaches are critical to ensuring vaccine R&D not only happens in an efficient, coordinated manner but also that such R&D yields production at scale. Moreover, it enabled a suite of approaches to vaccine development rather than one method, raising the overall probability of developing a successful vaccine.
Repeating these lessons in subsequent EID vaccine developments would generate both significant returns on investment and benefits to society.
Plan of Action
By incentivizing vaccine development for priority EIDs, the federal government can preemptively solve market failures without picking winners or losers.
First, Congress should authorize and appropriate $10 billion to BARDA over 10 years to create a Dynamic Vaccine Development Fund. This fund would build on BARDA’s unique competencies as an engagement platform with the private sector. would allow for new developments to emerge
It would also enact the following strategies, gleaned from all of which were proven to be effective in OWS:
- Advanced market commitments to purchase large quantities of vaccines in cases of an outbreak.
- Ensuring steady incremental progress in combatting the most dangerous EIDs.
- Supporting manufacturing and distribution facilities.
- Providing limited government guarantees, equities, and securities to investors funding vaccine programs for a pre-specified list of priority diseases.
As illustrated by its successful history, BARDA is well-positioned to manage a large-scale vaccine initiative. Last year, BARDA announced the first venture capital partnership with the Global Health Investment Corporation to “allow direct linkage with the investment community and establish sustained and long-term efforts to identify, nurture, and commercialize technologies that aid the U.S. in responding effectively to future health security threats.” During the COVID-19 pandemic, BARDA and Janssen shared the R&D costs to help move Janssen’s investigational novel coronavirus vaccine into clinical evaluation—a collaboration supported by their previous successes on the Ebola vaccine. The Government Accountability Office reported that BARDA had also supported scaled production by identifying additional manufacturing partners. This partnership record shows that BARDA not only knows how to manage global health projects to completion but also is particularly adept at interfacing with the private sector. As such, it stands out as an ideal manager for the Dynamic Vaccine Development Fund.
With $10 billion, this Fund could not only support the vaccine economy, but also save millions of lives and trillions of dollars. Although the price tag is admittedly hefty, it is reasonable. After all, OWS had a price tag of $12+ billion––a small investment compared to the $16+ trillion cost of COVID-19. As seen in OWS, the long-term benefits of upfront, robust financing are even more impactful. One back-of-the-envelope calculation suggests immense economic returns for the Fund:
- With a 50% chance of another $16 trillion COVID-like pandemic in the next 25 years, the expected cost over this timeframe is $8 trillion globally.
- One expected outcome of this Fund would be to prevent 31 of the next 45 pandemics, or a nearly 69% chance of preventing the next epidemic in expectation.
- A 69% chance of preventing an $8 trillion cost over the next 25 years would yield an expected value of $5.6 trillion globally.
A $10 billion down payment would allow the Fund to excel in its normal operations (see bulleted list above) and support up to 120 vaccine candidates. OWS also spawned more than just new breakthrough R&D in the use of mRNA vaccine models. It also led to a health and biotechnology innovation windfall:
“Now that we know that mRNA vaccines work, there is no reason we could not start the process of developing those for the top 20 most likely pandemic pathogen prototypes”
Dr. Francis Collins, former director of the National Institutes of Health
Ten billion dollars would ensure the Fund’s impact could be similarly force-multiplied by private sector partnerships. There would be more time available and more opportunity for creative partnerships with the private sector. The Fund’s purpose is to lower financial risks and attract large amounts of capital from the bond market, whose size outweighs the venture capital, public equity, or private equity markets. Indeed, there has been growing interest in the application of social bonds to pandemic preparedness as a unique instrument for rapidly frontloading resources from capital markets. Though this Fund will assume a different form, the International Finance Facility for Immunisation represents a proof of concept for coordinating philanthropic foundations, governments, and supranational organizations for the purpose of “raising money more quickly.” With seed capital, this Fund could provide a strong signal — and perhaps an anchor for coordination — to debt capital markets to make issuances for vaccines. To this end, the targeted critical mass of $10 billion is estimated to generate both tremendous societal value by preventing future epidemic outbreaks as well as producing positive returns for investors.
Second, in executing Fund activities, BARDA should leverage investment strategies––such as milestone-based payments––to incentivize maximum vaccine innovation. When combatting EIDs, the U.S. will need as many vaccine options as possible. To facilitate this outcome, vaccine manufacturers should be rewarded for producing multiple kinds of vaccines at the same time. For example, BARDA might support the development of vaccines for a given EID by funding progress for four novel methods (e.g., mRNA, recombinant protein, gene-therapy, and live attenuated, orally-administered vaccines).
Furthermore, these rewards should come regularly during major events––or “milestones”––during development. Initial-stage milestones include vaccine candidates that protect an animal model against disease; later-stage milestones include human clinical trials. This financing model would provide companies with clear, short-term targets, reducing uncertainty and rewarding progress dynamically. Additionally, it would support the recent executive order, which calls for “increasing piloting and prototyping efforts in biotechnology and biomanufacturing to accelerate the translation of basic research results into practice.”
BARDA could expand the milestone-based financing mechanism further by employing early-stage challenges. In this scenario, it would only fund the first two of three candidates that successfully complete small-scale clinical trials. The final milestone stage––which should only be offered to a limited number of candidates––should provide an advanced market commitment to house complete vaccines within U.S. storage facilities, based on the interagency effort (described in the paragraph below). The selections process would retain sufficient competition throughout the development process, while ensuring a sustainable method for scaling up certain vaccines based on mission priorities.
Third, to support Fund activities towards late-stage clinical trials, the White House Office of Science and Technology Policy (OSTP) should coordinate a larger-scale interagency effort leveraging advanced market commitments, prize challenges, and other innovative procurement techniques. OSTP should be a coordinator across federal agencies that address pandemic preparedness, which might include: the Department of Defense, BARDA, the U.S. Agency for International Development, the National Institute of Allergy and Infectious Diseases, the Federal Emergency Management Agency, and the Development Finance Corporation. In doing so, the OSTP can (i) consolidate investments for particular vaccine candidates, and (ii) utilize networks and incentive strategies across the U.S. government to secure vaccines. Separately––and based on urgent priorities shared by agencies––OSTP should work closely with the Food and Drug Administration (FDA) to explore opportunities for pre-approval of vaccines as they develop through the trial phase.
Conclusion
Vaccines are among the most powerful tools for fighting pandemics. Unfortunately, bringing vaccines to market at scale is challenging. However, Operation Warp Speed (OWS) established a new precedent for tackling vaccine innovation market failures, laying the groundwork for a new era of industrial strategy. Congress should take advantage and supercharge U.S. pandemic preparedness by enabling the Biomedical Advanced Research and Development Authority (BARDA) to build a Dynamic Vaccine Development Fund. Embracing lessons learned from OWS, the Fund would incentivize companies to create vaccines for the six emerging infectious diseases most likely to cause the next pandemic.
The regulatory process for approving vaccines is even more reason to develop them ahead of time—before they are needed, rather than after an outbreak. Having access to an effective vaccine even days sooner can save thousands of lives due to the exponential rate of growth of all infectious diseases. Moreover, the FDA approval process—especially its Emergency Use Authorization Program—is extremely efficient, and is not the bottleneck for vaccine development. The main delay involved in vaccine development is the time it takes to conduct randomized clinical trials. Unfortunately, there are no shortcuts to this process if we want to ensure that vaccines are safe and effective. That is why we need to develop vaccines before pandemics occur. The idea here is simply to develop the minimum viable product of vaccines for priority EIDs that positions these vaccines to rapidly scale in the event of a pandemic.
Yes, there are several examples of vaccine initiatives using this strategy. To list a few:
- The Coalition for Epidemic Preparedness Innovations (CEPI) has a “megafund” vaccine portfolio (i.e., they have 32 vaccine candidates as of April 2022). This portfolio spans 13 different therapeutic mechanisms and five different stages of clinical development, from preclinical to “Emergency Use Listing” by the World Health Organization.
- BridgeBio, Roivant Sciences have used portfolio-based approaches for drug development.
- The National Brain Tumor Society is also leveraging this approach to finance novel drug candidates that can treat glioblastoma.
Ideally, vaccines in the final milestone stage would be stored in the United States and in line with new CDC guidance in the Vaccine Storage and Handling toolkit. This prevents the scenario where vaccines are held up in transit due to complex international negotiations and, potentially, expire during the lengthy proceedings. This exact scenario occurred when the 300,000 doses of monkeypox vaccine held in a Denmark-based facility were slowly and inconsistently onshored back to the U.S.
In addition, vaccines that are financed through the Fund would not always be final products. Instead, they would potentially be at varying stages of development thanks to the milestone-based payment strategy and frequent progress reviews. This would make it easier for the federal government to closely coordinate vaccine development with manufacturing professionals and rapidly increase vaccine production if necessary. The strategy offered in this memo lowers the risk of a similar situation occurring again.
We recommend that the executive order on biomanufacturing continue exploring this issue and investigate ways to securely store completed vaccines. The Government Accountability Office, for example, recently suggested several promising and discrete changes to update the requirements and operations of the Strategic National Stockpile.
This list was derived from justifications listed on CEPI’s website, linked here.
There are simply too many infectious diseases in nature, and most of are too rare to pose a significant threat. It would be scientifically and financially impractical––and unnecessary––to develop vaccines against all of them. However, we can greatly increase our readiness by widening our scope and developing a library of prototyped vaccines based on the 25 viral families (as called for by CEPI). Doing so would allow us to respond quickly against even unlikely pandemic scenarios.
Masks via Mail: Maintaining Critical COVID-19 Infrastructure for Future Public Health Threats
Summary
To protect against future infectious disease outbreaks, the Department of Health and Human Services (HHS) Coordination Operations and Response Element (H-CORE) should develop and maintain the capacity to regularly deliver N95 respirator masks to every home using a mail delivery system. H-CORE previously developed a mailing system to provide free, rapid antigen tests to homes across the U.S. in response to the COVID-19 pandemic. H-CORE can build upon this system to supply the American public with additional disease prevention equipment––notably face masks. H-CORE can helm this expanded mail-delivery system by (i) gathering technical expertise from partnering federal agencies, (ii) deciding which masks are appropriate for public use, (iii) pulling from a rotating face-mask inventory at the Strategic National Stockpile (SNS), and (iv) centralizing subsequent equipment shipping and delivery. In doing so, H-CORE will fortify the pandemic response infrastructure established during the COVID-19 pandemic, allowing the U.S. government to face future pathogens with preparedness and resilience.
Challenge and Opportunity
The infrastructure put in place to respond to COVID-19 should be maintained and improved to better prepare for and respond to the next pandemic. As the federal government thinks about the future of COVID-19 response programs, it should prioritize maintaining systems that can be flexibly used to address a variety of health threats. One critical capability to maintain is the ability to quickly deliver medical countermeasures across the US. This was already done to provide the American public with COVID-19 rapid tests, but additional medical countermeasures––such as N95 respirators––should also be included.
N95s are an incredibly effective means of preventing deadly infectious disease spread. Wearing an N95 respirator reduces the odds of testing positive for COVID-19 by 83%, compared to 66% for surgical masks and 56% for cloth masks. The significant difference between N95 respirators and other face coverings means that N95 respirators can provide real public health benefits against a variety of biothreats, not just COVID-19. Adding N95 respirators to H-CORE’s mailing program would increase public access to a highly effective medical countermeasure that protects against a variety of harmful diseases. Providing equitable access to N95 masks can also protect the United States against other dangerous public health emergencies, not just pandemics. Additionally, N95s protect individuals from harmful, wildfire-smoke-derived airborne particles, providing another use-case beyond protection against viruses.
Beyond the benefit of expanding access to masks in particular, it is important to have an active public health mailing system that can be quickly scaled up to respond to emergencies. In times of need, this established mailing system could distribute a wide array of medical countermeasures, medicines, information, and personal protective equipment––including N95s. Thankfully, the agencies needed to coordinate this effort are already primed to do so. These authorities already have the momentum, expertise, and experience to convert existing COVID-19 response programs and pandemic preparedness investments into permanent health response infrastructure.
Plan of Action
The newly-elevated Administration for Strategic Preparedness and Response (ASPR) should house the N95 respirator mailing system, granting H-CORE key management and distribution responsibilities. Evolving out of the operational capacities built from Operation Warp Speed, H-CORE has demonstrated strong logistical capabilities in distributing COVID-19 vaccines, therapeutics, and at-home tests across the United States. H-CORE should continue operating some of these preparedness programs to increase public access to key medical countermeasures. At the same time, it should also maintain the flexibility to pivot and scale up these response programs as soon as the next public health emergency arises.
H-CORE should bolster its free COVID-19 test mailing program and include the option to order one box of 10 free N95 respirator masks every quarter.
H-CORE partnered with the U.S. Postal Service (USPS) to develop an unprecedented initiative––creating an online ordering system for rapid COVID-19 testing to be sent via mail to American households. ASPR should maintain its relationships with USPS and other shipping companies to distribute other needed medical supplies––like N95s. To ensure public comfort, a simple N95 ordering website could be designed to mimic the COVID-19 test ordering site.
An N95-distribution program has already been piloted and proven successful. Thanks to ASPR and the National Institute for Occupational Safety and Health (NIOSH), masks previously held at SNS were made available to the public at select retail pharmacies. This program should be made permanent and expanded to maximize the convenience of obtaining medical countermeasures, like masks. Doing so will likely increase the chance that the general population will acquire and use them. Additionally––if supplies are sourced primarily from domestic mask manufacturers––this program can stabilize demand and incentivize further manufacturing within the United States. Keeping this production at a steady base level will also make it easier to scale up quickly, should America face another pandemic or other public health crisis.
H-CORE and ASPR should coordinate with the SNS to provide N95 respirators through a rotating inventory system.
As evidenced by the 2009 H1N1 influenza pandemic and the COVID-19 pandemic, static stockpiling large quantities of masks is not an effective way to prepare for the next bio-incident.
Congress has long recognized the need to shift the stockpiling status quo within HSS, including within the SNS. Recent draft legislation––including the Protecting Providers Everywhere (PPE) in America Act and PREVENT Pandemics Act, as well as being mentioned in the National Strategy for a Resilient Public Health Supply Chain––have advocated for a rotating stock system. While the concept is mentioned in these documents, there are few details on what the system would look like in practice or a timeline for its implementation.
Ultimately, the SNS should use a rotating inventory system where its stored masks get rotated out to other uses in the supply chain using a “first in, first out” approach. This will prevent N95s from being stored beyond their recommended shelf-life and encourage continual replenishment of the SNS’ mask stockpile.
To make this new rotating inventory system possible, ASPR should pilot rotating inventory through this H-CORE mask mailing program while they decide if and how rotating inventory could be implemented in larger quantities (e.g. rotating out to Veterans Affairs, the Department of Defense, and other purchasers). To pilot a rotating inventory system, the Secretary of HHS may enter into contracts and cooperative agreements with vendors, through the SNS contracting mechanisms, and structure the contracts to include maintaining a constant supply and re-stock capacity of the stated product in such quantities as required by the contract. As a guide, the SNS can model these agreements after select pharmaceutical contracts, especially those that have stipulated similar rotating inventory systems (i.e., the radiological countermeasure Neupogen).
The N95 mail-delivery system will allow ASPR, H-CORE, and the SNS to test the rotating stock model in a way that avoids serious risk or negative consequences. The small quantity of N95s needed for the pilot program should not tax the SNS’ supply-at-large. After all, the afore-mentioned H-CORE/NIOSH mask-distribution programs are similarly designed to this pilot, and they do not disrupt the SNS supply for healthcare workers.
Conclusion
To be fully prepared for the next public health emergency, the United States must learn from its previous experience with COVID-19 and continue building the public health infrastructures that proved efficient during this pandemic. Widespread distribution of COVID-19 rapid diagnostic tests is one such success story. The logistics and protocols that made this resource dispersal possible should be continued for other flexible medical countermeasures, like N95 respirators. After all, while the need for COVID-19 tests may wane over time, the relevance of N95 respirators will not.
HHS should therefore distribute N95 respirators to the general public through H-CORE to (i) maintain the existing mailing infrastructure and (ii) increase access to a medical countermeasure that efficiently impedes transmission for many diseases. The masks for this effort should be sourced from the Strategic National Stockpile. This will not only prevent stock expiration, but also pilot rotating inventory as a strategy for larger-scale integration into the SNS. These actions will together equip the public with medical countermeasures relevant to a variety of diseases and strengthen a critical distribution program that should be maintained for future pandemic response.
Medical countermeasures (MCMs) can include both pharmaceutical interventions (such as vaccines, antimicrobials, antivirals, etc.) and non-pharmaceutical interventions (such as ventilators, diagnostics, personal protective equipment, etc.) that are used to prevent, mitigate, or treat the adverse health effects or a public health emergency. Examples of MCM deployment during the COVID-19 pandemic include the COVID-19 vaccines, therapeutics for COVID-19-hospitalized patients (e.g., antivirals and monoclonal antibodies), and personal protective equipment (e.g., respirators and gloves) deployed to healthcare providers and the public.
This proposal would build off of capabilities already being executed under the Department of Health and Human Services, Administration for Strategic Preparedness and Response (HHS ASPR). ASPR oversees both H-CORE and the Strategic National Stockpile (SNS) and was recently reclassified from a staff division to an operating division. This change allowed ASPR to better mobilize and respond to health-related emergencies. ASPR established H-CORE at the beginning of 2022 to create a permanent team responsible for coordinating medical countermeasures and strengthening preparedness for future pandemics. While H-CORE is currently focused on providing COVID-19 countermeasures––including vaccines, therapeutics, masks, and test kits––their longer-term mission is to augment capabilities within HHS to solve emerging health threats. As such, their ingrained mission and expertise match those required to successfully launch an N95 mail-delivery system.
Presently, 270 million masks have been made available to the U.S. population. It’s estimated that this same number of masks would be enough for American households to receive 10 masks per quarter, assuming a 50% participation rate in the program.
The total annual cost of this program is an estimated $280 million to purchase 270 million masks and facilitate shipping across the United States.
There are several ways this initiative could be funded. Initial funding to purchase and mail COVID-19 tests to homes came from the American Rescue Plan. By passing the COVID Supplemental Appropriations Act, Congress could provide supplemental funds to maintain standing COVID-19 programs and help pivot them to address evolving and future health threats.
The FY2023 President’s Budget for HHS also provides ample funding for H-CORE, the SNS, and ASPR, meaning it could also provide alternative funding for an N95 mail-delivery system. Presently, the budget asks for: $133 million for H-CORE and mentions their role in making masks available nationwide. Additionally, $975 million has been allotted to the SNS, which includes coordination with HHS and maintaining the stockpile. Furthermore, is petitions for ASPR to receive $12 billion to generally prepare for pandemics and other future biological threats (and here it also specifically recommends strong coordination with HHS agency efforts).
N95 respirators have a number of benefits that make them a critical defense strategy in a public health emergency. First, they are pathogen-agnostic, shelf-stable countermeasures that filter airborne particles very efficiently, meaning they can impede transmission for a variety of diseases––especially airborne and aerosolized ones. This is important, since these two latter disease categories are the most likely naturally occurring and intentional biothreats. Second, N95 respirators are useful beyond pandemic responses and also protect against wildfire smoke. Additionally, N95 masks have a long shelf-life. Therefore, the ability to quickly and widely distribute N95s is a critical public health preparedness measure.
Domestic mask manufacturers have also frequently experienced boom and bust cycles as public demand for masks can change rapidly and without warning. This inconsistent market makes it difficult for manufacturers to invest in increased manufacturing capacity in the long-term. One example is the company Prestige Ameritech, which invested over $1 million in new equipment and hired 150 new workers to produce masks in response to the 2009 swine flu outbreak. However, by the time production was ready, demand for masks had dropped and the company almost went bankrupt. Given overwhelmingly positive benefits of having mask manufacturing capacity available when needed, it is worthwhile for the government to provide some ongoing demand certainty.
Furthermore, making masks free and easily available to the general public could increase the public’s mask usage during the annual flu season and other periods of sickness. While personal protective equipment has decreased in cost since the peak of the pandemic, making them as accessible as possible will disproportionately increase access for low-income citizens and help ensure equitable access to protective medical countermeasures.
It is true that N95s are not regulated outside of healthcare settings, but that shouldn’t dissuade public use. Presently, there is no federal agency currently tasked with regulating respiratory protection for the public. The Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) National Institute for Occupational Safety and Health (NIOSH) currently have a Memorandum of Understanding (MOU) coordinating regulatory authority over N95 respirators for medical use. Neither the FDA nor NIOSH, though, have jurisdiction of mask use in a non-medical, non-occupational setting. Using an N95 respirator outside of a medical setting does not satisfy all of the regulatory requirements, like undergoing a fit-test to ensure proper seal. However, using N95 respirators for every-day respiratory protection (i) provides better protection than no mask, a cloth mask, or a surgical mask, and (ii) realistically should not need to meet the same regulatory standards as medical use as people are not regularly exposed to the same level of risk as medical professionals.
Presently, there is no central regulator for public respiratory protection in general. In fact, the National Academies of Science Engineering and Medicine recently issued a recommendation for Congress to “expeditiously establish a coordinating entity within the Department of Health and Human Services (HHS) with the necessary responsibility, authority, and resources (financial, personnel, and infrastructure) to provide a unified and authoritative source of information and effective oversight in the development, approval, and use of respiratory protective devices that can meet the needs of the public and protect the public health.”
Moving forward, NIOSH alone should regulate N95 use for the public just as they do in occupational settings. The approval process used by other regulators––like the FDA––is more restrictive than necessary for public use. The FDA’s standards for medical protection understandably need to be high in order to protect doctors, nurses, and other medical professionals against a wide variety of dangerous exposure situations. NIOSH can provide alternative regulation and guidance for the general public, who realistically are unlikely to be in similar circumstances.
Aside from federal agencies, professional scientific societies have also provided their input in regulating N95s. The American Society for Testing and Materials (ASTM), for example, recently published standards for barrier face coverings not intended for medical use or currently regulated under NIOSH standards. While ASTM does not have any regulatory or enforcement authority, HHS could use these standards for protection, comfort, and usability as a starting point for developing guidelines for respirators suitable for public distribution and use.
After the 2009 H1N1 influenza pandemic and the COVID-19 pandemic, it became evident that SNS must change its stockpile management practices. The stockpile’s reserves of N95 respirators were not sufficiently replenished after the 2009 H1N1 pandemic, in large part due to the significant up-front supply restocking cost. During the early days of COVID-19 response, many states received expired respirators and broken ventilators from the SNS. These incidents revealed a number of issues with the current stockpiling paradigm. Shifting to a rotating inventory system would prevent issues with expiration, smooth out the costs of large periodic restocks, and help maintain a capable and responsive manufacturing base.
Addressing the Mental Health Crisis Among Predoctoral and Postdoctoral Researchers in STEM
Summary
The growing mental–health crisis among science, technology, engineering, and math (STEM) doctoral and postdoctoral researchers threatens the future and competitiveness of science and technology in the United States. The federal government should tackle this crisis through a four-part approach to (i) improve data collection on the underlying drivers of mental-health struggles in STEM, (ii) discourage behaviors and cultures that perpetuate stress, (iii) require Principal Investigators (PIs) to submit a statement of their mentoring philosophy as part of applications for federally supported research grants, and (iv) increase access to mental-health care for predoctoral and postdoctoral researchers.
Challenge and Opportunity
The prevalence of mental-health problems is higher among Ph.D. students than in the highly educated general population: fully half of Ph.D. students experience psychological distress. In a survey of postdoctoral researchers conducted by Nature, 51% of respondents reported considering leaving science due to work-related mental-health concerns. 65% of respondents reported experiencing power imbalances or bullying during their postdoctoral appointments, and 74% reported observing the same. Stress accumulation not only leads to the development of neuropsychiatric disorders among the developing STEM workforce — it also contributes to burnout. At a time when advancing U.S. competitiveness in science and technology is of utmost importance, the mental-health crisis is depleting our nation’s STEM pipeline when we should be expanding and diversifying it. This is a crisis that the federal government is well-positioned to and must solve.
Plan of Action
The federal government should counter the mental-health crisis for U.S. doctoral and postdoctoral researchers through a four-part approach to (i) improve data collection on the underlying drivers of mental-health struggles in STEM, (ii) discourage behaviors that perpetuate stress, (iii) require PIs to submit a statement of their mentoring philosophy as part of applications for federally supported research grants, and (iv) increase access to mental-health care for doctoral and postdoctoral researchers. Detailed recommendations associated with each of these steps are provided below.
Part 1. Improve data collection
Data drives public policy. Various organizations conduct surveys evaluating the mental health of doctoral and postdoctoral researchers in STEM, but survey designs, target audiences, and subsequent follow-up and monitoring are inconsistent. This fragmented information ecosystem makes it difficult to integrate and act on existing data on mental health in STEM. To provide a more comprehensive picture of the STEM mental-health landscape in the United States, the National Institutes of Health (NIH) and the National Science Foundation (NSF) should work together to conduct and publish biennial evaluations of the state of mental health of the STEM workforce. The survey format could be modeled on the NSF’s Survey of Doctorate Recipients or the Survey of Earned Doctorates — and, like those surveys, resultant data could be maintained at NSF under the National Center for Science and Engineering Statistics. Once established, the data from the survey can be used to track effectiveness of programs that are implemented and direct the federal government to change or start new initiatives to modify the needs of doctoral and postdoctoral researchers. Additionally, the NSF and NIH could partner with physicians within HHS to define and establish what “healthy” means in terms of mental-health guidelines in order to establish new program guidelines and goals.
Part 2. Discourage problematic behaviors
The future of a doctoral or postdoctoral researcher depends considerably on the researcher’s professional relationship with their PI(s). Problems in the relationship — including bullying, harassment, and discrimination — can put a trainee in a difficult situation, as the trainee may worry that confronting the PI could compromise their career opportunities. The federal government can take three steps to discourage these problematic behaviors by requiring PIs to submit and implement training and mentorship plans for all grant-supported trainees.
First, the White House Office of Science and Technology Policy (OSTP) should assemble a committee of professionals in psychology, social sciences, and human resources to define what behaviors constitute bullying and harassment in academic work environments. The committee’s findings should be publicized via a web portal (similar to NSF’s website on Sexual Harassment), and included in all requests for grant applications issued by federal STEM-funding agencies (in order to raise awareness among PIs).
Second, federal STEM-funding agencies should require universities to submit annual reports of bullying to federal, grant-issuing agencies. NSF already requires institutions to report findings of sexual harassment and other forms of harassment and can revoke grants if a grantee is found culpable. NSF and other STEM-funding agencies should add clarity to this definition and broaden this reporting to include bullying and retaliation to include bullying and retaliation attempts by PIs, with similar consequences for repeated offenses. Reinstatement of privileges (e.g., reinstatement of eligibility for federal grant funding) would be considered on a case-by-case basis by the grant-issuing institution and could be made contingent on implementation of an adequate “re-entry” plan by the PI’s home institution. The NIH Office of Behavioral and Social Science Research should be consulted to help formulate such “re-entry” plans to benefit both researchers and PIs.
Third, STEM-funding agencies could work together to establish a mechanism whereby trainees can anonymously report problematic PI behaviors. NSF has a complaint form for those who wish to report incidents for incidents of sexual harassment or harassment. Thus, NSF could expand their system to accept broader incidents such as bullying and retaliation attempts and NIH could use this complaint form as a template for reporting as well. In conjunction with reporting misconduct, a “two-strike” accountability system should be imposed if a PI is found guilty of harassment, bullying, or other behaviors that could contribute to the development of a neuropsychiatric disorder. After receiving a first strike (report of problematic behavior and a guilty verdict), the PI would be given a warning and be required to participate in relevant training workshops and counseling using a plan outlined by social science professionals at NIH. If a second strike is received, the PI would lose privileges to apply for federal grant funding and opportunities to serve on committees that are often favored for tenure and promotion, such as grant review committees. Again, reinstatement of privileges would be considered on a case-by-case basis by the grant-issuing institution and could be made contingent on implementation of an adequate “re-entry” plan.
Part 3. Require submission of mentoring philosophies
NIH F31 predoctoral and F32 postdoctoral award applications already require PIs to submit mentoring plans for their trainees to receive professional-development training. Federal STEM-funding agencies should build on this precedent by requiring PIs applying for federal grants to submit not just mentoring plans, but brief summaries of their mentoring philosophies. As the University of Colorado Boulder explains, a mentoring philosophy
“…defines [a mentor’s] approach to engaging with students as [they] guide their personal growth and professional development, often explaining [the mentor’s] motivation to mentor with personal narratives while highlighting their goals for successful relationships and broader social impact. These statements may also be considered ‘living documents’ that are updated as [the mentor] refine[s[ [their] approach and the context and goals of [their] work changes.”
Mentoring philosophies help guide development of and updates to individualized mentoring plans. Mentoring philosophies also promote equity and inclusion among mentees by providing a common starting point for communication and expectations. Requiring PIs to create mentoring philosophies will elevate mental health among doctoral and postdoctoral researchers in STEM by promoting effective top-down mentorship and discouraging unintended marginalization. And since a growing number of university faculty are already creating mentoring philosophies, this new requirement shouldn’t be seen as just another administrative burden; rather, it would serve as a means to quickly perpetuate a best practice that is already spreading. The federal government can support PIs in adhering to this new requirement by working with external partners to collect and broadly share resources related to preparing mentoring philosophies. The Center for the Improvement of Mentored Experiences in Research, for instance, has already assembled a suite of such resources on its web platform.
Part 4. Increase access to mental health care
Concurrent with reducing causes of mental health burdens, the federal government should work to expand doctoral and postdoctoral researchers’ access to adequate mental-health care. Current access may vary considerably depending on the level of insurance coverage offered by a researcher’s home institution. Inspired by legislation (S. 3048 – Stopping the Mental Health Pandemic Act, where funds can be used to support and enhance mental health services) introduced in the 117th Congress, the Department of Health and Human Services (HHS) should partner with federal STEM-funding agencies to design and implement new pathways, programs, and opportunities to strengthen mental-health care among early-career STEM professionals. In particular, the federal government could create a library of model policies that federally funded public and private institutions could adopt to strengthen mental-health care for employed early-career researchers. Examples include allowing trainees to take time off during the workday to receive mental-health treatment without expectations to make up hours outside of business hours, providing a supplemental stipend for trainees to pay for therapy costs that are not covered by insurance, and addressing other sources of stress that can exacerbate stressful situations, such as increasing stipends to decrease financial stress.
Conclusion
The U.S. science and technology enterprise is only as strong as the workforce behind it. Failing to address the mental-health crisis that plagues early-career researchers will lead the United States to fall behind in global research and development due to talent attrition. President Biden’s 2022 State of the Union address cited mental health as a priority area of concern. There is an especially clear need for a culture change around mental health in academia. The four actions detailed in this memo align with the President’s policy agenda. By improving data collection on the mental-health status of STEM doctoral and postdoctoral researchers, discouraging behaviors and cultures that produce stress among this population, improving training and mentorship at universities, and expanding access to mental-health care among STEM doctoral and postdoctoral researchers, the federal government can ensure that success for early-career STEM professionals does not demand mental-health sacrifice.
STEM fields are closely tied to the U.S. economy, supporting two-thirds of U.S. jobs and 69% of the U.S. Gross Domestic Product (GDP). Attrition of U.S. researchers from STEM fields due to mental-health challenges has disproportionately adverse effects on American society and undermines U.S. competitiveness. Policymakers should prioritize actions designed to combat the mental-health crisis in STEM.
NSF already requires that universities who receive federal research funding conduct internal investigations to validate claims of harassment and sexual harassment. Similar policies could be implemented regarding reported bullying and/or workplace harassment. If an allegation is found to be false, it should be handled by university-specific policies.
The goal of requiring PIs to attend workshops on mentorship and therapy sessions is to help them better themselves and improve their ability to mentor the next generation of STEM professionals. Re-entry to mentoring trainees will be closely monitored by leadership faculty who should conduct surveys of both mentors and mentees to determine if the PI understands (a) their previous misconduct and (b) the lasting mental health effects that their previous actions inflicted on their trainees.
NIH and NSF are arguably the two leading federal agencies when it comes to providing federal funding for graduate students. That said, recommendations presented in this memo could easily be extended to other STEM-funding agencies. For instance, there is a timely opportunity to extend these recommendations to the Department of Energy (DOE). DOE is currently working to manage the President’s major FY23 investment in clean energy and sustainability, including through significant research-grant funding. Coupling these new grants with policies designed to mitigate mental-health burdens among early-career researchers could help foster a more resilient and productive clean-energy workforce and serve as a pilot group for the NIH and NSF to follow.
The administrative responsibilities for reporting are minimal. NSF’s Organizational Notification of Harassment Form can — at a minimum — be used as a template for NSF, NIH, and other agencies to notify the federal government of guilty verdicts from universities. Alternatively, doctoral and postdoctoral researchers can submit incidents for reporting by federal agencies similar to NSF’s existing complaint form, which would reduce the initial administrative burden of university employees but may create additional hours of work once federal agencies conduct their investigations.
While the strategies above teach researchers how to cope with stress, a long-term, more supportive approach would be to reduce stress by going straight to the source. Actions such as addressing harassment and bullying will benefit not only the researcher themselves, but others in the work environment by fostering a responsible, low-stress culture.
7. How are mentoring philosophies different from mentoring plans?
The submission of mentoring plans by PIs are currently required for NIH pre- and post-doctoral fellowship applications. They are meant to supplement the training of a researcher by focusing on the logistics of skill building. However, mentorship of a researcher transcends knowledge and skill-building — it also encompasses the holistic development of a researcher, supporting and respecting their interests, values, and considerations of their individual situations. Thus, submission of a mentoring philosophy is meant to stimulate thoughts and conversations about how a PI wants to communicate openly and honestly with their trainee and how they can adapt to support the mentoring style that best fits their trainee.
Establishing a National Endemic Disease Surveillance Initiative (NEDSI)
Summary
Global pandemics cause major human and financial losses. Our nation has suffered nearly a million deaths associated with COVID-19 to date. The Congressional Budget Office estimates that COVID-19 will cost the United States $7.6 trillion in lost economic output over the next decade. While much has rightly been written on preventing the next pandemic, far less attention has been paid to mitigating the compounding effects of endemic diseases. Endemic diseases are consistently present over time and typically restricted to a defined geographic region. Such diseases can exacerbate pandemic-associated financial losses, complicate patient care, and delay patient recovery. In a clinical context, endemic diseases can worsen existing infections and compromise patient outcomes. For example, co-infections with endemic diseases increase the likelihood of patient mortality from pandemic diseases like COVID-19 and H1N1 influenza.
Accurate and timely data on the prevalence of endemic diseases enables public-health officials to minimize the above-cited burdens through proactive response. Yet the U.S. government does not mandate reporting and/or monitoring of many endemic diseases. The Biden-Harris administration should use American Rescue Plan funds to establish a National Endemic Disease Surveillance Initiative (NEDSI), within the National Notifiable Disease Surveillance System (NNDSS), to remove barriers to monitoring endemic, infectious diseases and to incentivize reporting. The NEDSI will support the goals of the Centers for Disease Control and Prevention (CDC)’s Data Modernization Initiative by providing robust infection data on a typically overlooked suite of diseases in the United States. Specifically, the NEDSI will:
- Provide healthcare practitioners with resources to implement/upgrade digital disease reporting.
- Support effective allocation of funding to hospitals, clinics, and healthcare providers in regions with severe endemic disease.
- Prepare quarterly memos updating healthcare providers about endemic disease prevalence and spread.
- Alert citizens and health-care practitioners in real time of notable infections and disease outbreaks.
- Track and predict endemic-disease burden, enabling strategic-intervention planning within the CDC and with partner entities.
Challenge and Opportunity
The COVID-19 pandemic highlighted the need for a multilevel approach to addressing endemic diseases. Endemic diseases are defined as those that persist at relatively stable case numbers within a defined geographic region. Though endemic diseases are typically geographically restricted, changes in population movement, population behaviors, and environmental conditions are increasing the incidence of endemic diseases. For example, Valley fever, a fungal respiratory disease endemic to the California Central Valley and the American Southwest, is predicted to spread to the American Midwest by 2060 due to climate change.
Better preparing the United States for future pandemics depends partly on better countering endemic disease. Effective patient care during a pandemic requires clinicians to treat not only the primary infection, but also potential secondary infections arising from endemic pathogens taking advantage of a weakened, preoccupied host immune system. Though typically not dangerous on their own, secondary infections from even common fungi such as Aspergillus or Candida can become deadly if the host is pre-infected with a respiratory virus. On the individual level, secondary infections with endemic diseases adversely impact patient recovery and survival rates. On the state level, secondary infections impose major healthcare costs by prolonging patient recovery and increasing medical intervention needs. And on the national level, poor endemic-disease management in one state can cause disease persistence and spread to other states.
Robust surveillance is integral to endemic-disease management. The case of endemic schistosomiasis in the Sichuan province of China illustrates the point. Though the province successfully controlled the disease initially, decreased funding for disease tracking and management—and hence lack of awareness and apathy among stakeholders—caused the disease to re-emerge and case numbers to grow. During active endemic-disease outbreaks, comprehensive data improves decision-making by reflecting the real-time state of infections. In between outbreaks, high-quality surveillance data enables more accurate prediction and thus timely, life-saving intervention. Yet the U.S. government mandates reporting and/or monitoring of relatively few endemic diseases.
Part of the problem is that improvements are needed in our national infrastructure for tracking and reporting diseases of concern. Approximately 95% of all hospitals within the United States use some form of electronic health record (EHR) keeping, but not all hospitals have the same resources to maintain or use EHR systems. For example, rural hospitals generally have poorer capacity to send, receive, find, and integrate patient-care reports. This results in drastic variation in case-reporting quality across the United States: and hence drastic variation in availability of the standardized, accurate data that policy and decision makers need to maximize public health.
With these issues in mind, the Biden-Harris administration should use American Rescue Plan (ARP) funds to establish a National Endemic Disease Surveillance Initiative (NEDSI) within the CDC’s National Notifiable Disease Surveillance System (NNDSS). Fighting an individual pandemic disease is difficult enough. We need better systems to stop endemic diseases from making the battle worse. Implementing NEDSI will equip decision makers with the data they need to respond to real-time needs— thereby protecting our nation’s economy and, more importantly, our people’s lives.
Plan of Action
To build NEDSI, the CDC should use a portion of the $500 million allocated in the ARP to strengthen surveillance and analytic infrastructure and build infectious-disease forecasting systems. NEDSI will support the goals of the CDC’s Data Modernization Initiative by allocating resources to implement and/or upgrade digital-disease reporting capabilities needed to obtain robust infection data on endemic diseases. Specifically, NEDSI would strive to minimize healthcare burdens of endemic diseases through the following four actions:
- Disease monitoring. NEDSI will identify and track notable endemic infectious diseases for each state, including but not exclusive to (i) existing infectious diseases with historical presence and/or relevance, and (ii) infectious diseases that disproportionately impact particular workers. For example, Valley fever disproportionately impacts those employed in outdoor occupations related to ground/soil work (such as agricultural workers, solar farmers, construction workers, etc.). Endemic-disease reporting under NEDSI will follow reporting templates and frameworks that have already been developed by the NNDSS, but will also include information on co-infections (i.e., whether a reported endemic-disease case was a primary, secondary, or higher-order infection).
- Disease notification. As part of monitoring, case-report numbers that rise above historical norms will be automatically flagged for alerts to community members, health-care providers, public-health officials, and other stakeholders.
- Alerts to community members will be geotargeted (for example, by city, county, or region), enabling residents and travelers in endemic zones to take precautions. Alerts will be text-message-based and include resource links vetted by public-health experts.
- Alerts to health-care providers will contain links to resources providing the latest information on accurate diagnosis and appropriate treatment of the disease in question. This will allow providers to quickly identify emerging cases of the disease, as well as to prepare for above-average use/need of particular treatments and equipment.
- Alerts to public-health officials will help shape recommendations for travel restrictions, emergency-funding requests and allocations, and rapid-response resources.
- Disease prediction. NEDSI will work with the CDC and the National Institutes of Health (NIH) to build an endemic-disease prediction model that ranks the severity of current and anticipated endemic-disease burden by geographic region in the United States, enabling proactive intervention against emerging threats.
- Model insights will be shared with the Federal Emergency Management Agency (FEMA) and state health departments to inform allocation of funds (e.g., from the federal-to-state and state-to-county levels) to support public health.
- Key model insights could also be posted on the CDC’s website and transmitted in notices to regional public-health officials and healthcare practitioners, especially when predicted risks and infection trends are high.
- Data underlying the model should be made publicly available and accessible to support external disease-modeling and -prediction efforts.
- In alignment with priorities of the Data Modernization Initiative and the American Pandemic Preparedness Plan, the CDC could also consider offering financial assistance (e.g., through grants or cooperative agreements) to external research efforts conducted in partnership with NEDSI and/or using NEDSI data. NEDSI and NNDSS should work to identify key research targets and promote them appropriately in Notices of Funding Opportunities.
- Health education. The NNDSS, utilizing data and model outputs from NEDSI, should prepare quarterly memos synthesizing key information related to endemic diseases in the United States, including (i) summary statistics of endemic-disease case numbers and co-infections by state and county; (ii) an up-to-date list of available treatments, medications, and therapies for different endemic diseases, and (iii) predicted disease trends for coming months and years. Memos should be published digitally and archived on the CDC website. Publication of each memo should be accompanied by a digital campaign to help spread the resource to healthcare practitioners, public-health authorities, and other stakeholders. NEDSI representatives should also prioritize participation in disease-specific research/clinical conferences to ensure that the latest scientific findings and developments are reflected in the memos.
Conclusion
Despite the clear burdens that endemic diseases impose, such diseases are still largely understudied and poorly understood. Until we have better knowledge of immunology related to endemic-disease co-infections, our best “treatment” is robust surveillance of opportunistic co-infections—surveillance that will enable proactive steps to minimize endemic-disease impacts on already vulnerable populations. Establishing a National Endemic Disease Surveillance Initiative within the National Notifiable Disease Surveillance System will close a critical gap in our nation’s disease-monitoring and -reporting infrastructure, helping reduce healthcare burdens while strengthening pandemic preparedness.
NEDSI, like other systems standardizing and streamlining disease reporting, will allow healthcare practitioners to efficiently—and in some cases, automatically—share data on endemic diseases. Such real-time, consistent data are invaluable for informing public-health responses as well as future emergency planning.
An ounce of endemic-disease prevention is worth far more than a pound of cure—and effective prevention depends on effective monitoring. Research shows that endemic diseases account for an alarming number of co-infections with COVID-19. These co-infections have detrimental impacts on patient outcomes. Further, population growth and migration trends are increasing transmission of and exposure to endemic diseases. Mitigating the severity of future epidemics and pandemics hence requires near-term investment in endemic-disease monitoring.
Yes: even in non-pandemic times, co-infections represent a major risk for the immunocompromised and elderly. AIDS patients succumb to secondary infections as a direct result of becoming immunocompromised by their primary HIV infection. Annual flu seasons are worsened by opportunistic co-infections. Monitoring and tracking endemic diseases and their co-infection rates will help mitigate existing healthcare burdens even outside the scope of a pandemic.
Due to a combination of funding challenges and lack of research progress/understanding, endemic-disease monitoring was only recently identified as a crucial gap in overall infectious disease preparedness. But now, with allocated funds from the American Rescue Plan to strengthen surveillance and infectious-disease forecasting systems, there is a historic opportunity to invest in this important area
Eliminating Childhood Lead Poisoning Worldwide
An estimated 815 million children (one in three) around the globe have dangerous levels of lead in their bloodstream, levels high enough to cause irreversible brain damage and impose severe health, economic, and societal consequences. 96% of these children live in low- and middle-income countries (LMICs), where collectively only about $6–10 million from non-governmental organizations is available each year to address the problem. To help eliminate childhood lead poisoning worldwide, the U.S. Federal Government should (1) add blood lead level (BLL) testing to the USAID-led Demographic and Health Survey Program, (2) create a Grand Challenge for Development to end childhood lead poisoning, and (3) push forward a global treaty on lead control.
Challenge and Opportunity
Lead is a potent toxin that causes irreversible harm to children’s brains and vital organs. Elevated body lead levels result in reduced intelligence, lower educational attainment, behavioral disorders, violent crime, reduced lifetime earnings, anemia, kidney disease, and cardiovascular disease. Impacts of lead on cognitive development are estimated to cause nearly $1 trillion of income loss in LMICs annually. Adverse health effects related to lead poisoning account for 1% of the global disease burden, causing 1 million deaths annually and substantial disability.
This enormous burden of lead poisoning in LMICs is preventable. It results from a combination of sources of exposure, some of the most important being:
- Lead that is intentionally added to paint, spices, cookware, and cosmetics.
- Lead that contaminates the environment from unsafe lead-acid battery and e-waste recycling practices.
- Lead that contaminates drinking water from pipes.
These sources of lead exposure have been effectively regulated in the United States and other high-income countries, which have seen average blood lead levels in their populations decline dramatically over the last 40 years. To achieve the same success, LMICs will need to prioritize policies such as:
- Regulation limiting the lead content of paint available on the market.
- Regulation of lead-acid battery and e-waste recycling.
- Inclusion of lead parameters in national drinking-water-quality standards.
- Regulation of the use of lead compounds in other locally important sources, such as spices, ceramics, cookware, toys, and cosmetics.
LMICs generally face three major barriers to implementing such policies:
- Lack of data on blood lead levels and on the scale and severity of lead poisoning. Most LMICs have no studies measuring blood lead levels. Policymakers are therefore unaware of the extent of the problem and hence unlikely to act in response.
- Lack of data on which sources of lead exposure are the biggest local contributors. Causes of lead poisoning vary spatially, but the vast majority of LMICs have not conducted source-apportionment studies. This makes it difficult to prioritize the most impactful policies.
- Limited access to equipment needed to detect lead in paint, spices, water, other sources, or the environment. Without needed detection capabilities, regulators cannot investigate the lead content of potential sources, nor can they monitor and enforce regulation of known sources.
These barriers are relatively simple to overcome, and when they are overcome do indeed result in action. As an example, at least 20 LMICs introduced legally binding lead paint regulation after the Global Alliance to Eliminate Lead Paint and its partners helped those countries confirm that lead paint was an important source of lead poisoning. Moreover, addressing childhood lead poisoning is in line with the priorities of the Biden Administration and the U.S. Agency for International Development (USAID). The Administration has already proposed an ambitious $15 billion plan to address childhood lead poisoning in the United States by eliminating lead pipes and service lines. By contributing to global elimination efforts (for only a fraction of what it will cost to solve the problem domestically), the Administration can multiply its impact on reducing childhood lead poisoning. Doing so would also advance USAID’s mission of “advanc[ing] a free, peaceful, and prosperous world”, since a reduction in childhood lead poisoning worldwide would improve health, strengthen economies, and prevent crime and conflict.
Plan of Action
Lead poisoning, from a variety of sources, affects one in three children worldwide. This is an unacceptable situation that demands action. The United States should adopt a three-part roadmap to help LMICs implement and enforce policies needed to achieve global elimination of childhood lead poisoning.
Recommendation 1. Add blood lead level (BLL) testing to the USAID-led Demographic and Health Survey.
USAID, through its Demographic and Health Survey (DHS), is in an ideal position to address the first barrier that LMICs face to implementing anti-lead poisoning policies: lack of data and awareness. The DHS collects, analyzes, and disseminates accurate and representative data on health in over 90 countries. Including BLL testing in the DHS would:
- Make accurate and representative data on the prevalence and severity of lead poisoning in LMICs available for the first time.
- Draw national and international attention to the immense burdens that childhood lead poisoning continues to impose.
- Determine which LMIC populations are most impacted by childhood lead poisoning.
- Motivate interventions to target the most impacted populations and most important sources of exposure.
- Support quantitative evaluation of interventions that aim to reduce lead exposure.
As such, USAID should add BLL testing of children into the DHS Biomarker Questionnaire for all host countries. This could be done in DHS revision for Phase 9, beginning in 2023. Including BLL testing in the DHS is also the first step to addressing the second barrier that LMICs face: lack of data on sources of lead exposure. BLL data collected through the DHS would reveal which countries and populations have the greatest lead burdens. These data can be leveraged by researchers, governments, and NGOs to investigate key sources of lead exposure.
BLL testing of children is feasible to carry out in the context of the DHS. It was successfully piloted in 1998 and 2002 via the DHS presence in India and Uzbekistan, but not rolled out further. Testing can be carried out using finger-stick capillary sampling and portable analyzers, so venipuncture and laboratory analysis are not required. Further, such testing can be carried out by health technicians who are already trained in capillary blood testing of children for anemia as part of the DHS. The testing can be conducted while questionnaires are administered, and results and any follow-up actions can be shared with the parent/guardian immediately. Alternatively, laboratory lead tests can be added onto sample analysis if blood draws are already being taken. Costs are low in both cases, estimated at around $10 per test.
Recommendation 2. Create a Grand Challenge for Development to end childhood lead poisoning.
Childhood lead poisoning in LMICs is dramatically neglected relative to the scale of the problem. Though childhood lead poisoning costs LMICs nearly $1 trillion annually and accounts for 1% of the global disease burden, only about $6–10 million per year is dedicated to addressing the problem. A USAID-led Grand Challenge for Development to end childhood lead poisoning would mobilize governments, companies, and foundations around generating and implementing solutions. In particular, the Challenge should encourage solutions to the second and third barriers presented above: lack of data on sources of lead exposure and limited detection capacity.
Recommendation 3. Push forward a global treaty on lead control.
A global push is needed to put childhood lead poisoning on the radar of decision-makers across the world and spur implementation and enforcement of policies to address the issue. The Biden Administration should lead an international conference to develop a global treaty on lead control. Such a treaty would set safe standards for lead in a variety of products (building on the Global Alliance to Eliminate Lead Paint’s toolkit for establishing lead-paint laws) and recommend regulatory measures to control sources of lead exposure. The success of the UN’s Partnership for Clean Fuels and Vehicles in bringing about global elimination of leaded gasoline illustrates that international political will to act can indeed be generated around lead pollution.
Conclusion
By implementing this three-part roadmap the Biden administration and USAID can make a historic and catalytic contribution towards global elimination of lead poisoning. There is true urgency; the problem becomes harder to solve each year as more lead enters the environment where it will remain a source of exposure for decades to come. Acting now will improve the health, wellbeing and potential of hundreds of millions of children.
Though relatively little investigation has been done on childhood lead poisoning in LMICs, the studies that do exist have consistently shown very high levels of lead poisoning. A recent systematic reviewidentified studies of background levels of childhood lead exposure in 34 LMICs. According to the review, “[o]f the 1.3 billion children (aged 0–14 years) living in the 34 LMICs with acceptable data on background blood lead levels in children, approximately 632 million…were estimated to have a level exceeding the CDC [Centers for Disease Control and Prevention] reference value of 5 μg/dL, and 413 million…were estimated to exceed the previous reference value of 10 μg/dL.” Data collected by the Institute of Health Metrics and Evaluation and analyzed in a joint UNICEF/Pure Earth report published in 2020 similarly concluded that dangerously elevated BLLs affect over 800 million children worldwide.
Major sources of lead poisoning in LMICs include paint, spices, cookware, pottery, pipes, cosmetics, toys, unsafe lead-acid battery recycling, unsafe e-waste recycling, and poorly controlled mining and smelting operations. High-income countries like the United States have relatively low levels of lead poisoning due to strong regulations around these sources of lead poisoning. Most high-income countries have, for instance, banned lead in gasoline and paint, set enforceable standards around the lead content of water, and imposed strong regulations around food adulteration. As a result, median BLLs in high-income countries have declined dramatically (in the United States, from 15ug/dL in the 1970s to <1µg/dL today). LMICs generally lack many of these effective controls around lead exposure and therefore have very high levels of childhood lead poisoning.
The most important thing that can be done to tackle the scourge of childhood lead poisoning is to impose source controls that prevent lead from entering the environment or consumer products. Though the relative contributions of different sources to childhood lead poisoning differ by country, effective policies and interventions tend to include:
- Regulations limiting the lead content of paint available on the market.
- Regulation of lead-acid battery and e-waste recycling practices.
- Inclusion of lead parameters in national drinking-water-quality standards.
- Regulation of the use of lead compounds in other locally important sources, such as spices, ceramics, cookware, toys, and cosmetics.
To enforce these policies, LMICs need testing capacity sufficient to monitor lead levels in potential exposure sources and in the environment. LMICs also need BLL monitoring to track the impact of policies and interventions. Fully eliminating childhood lead poisoning will ultimately involve abatement: i.e., removing lead already in the environment, such as by taking off lead paint already on walls and by replacing lead pipes. However, these interventions are extremely costly, with much lower impact per dollar than preventing lead from entering the environment in the first place.
An extreme lack of awareness, lack of data, and lack of advocacy around childhood lead poisoning in LMICs has created a vicious cycle of inattention. A large part of the problem is that lead poisoning is invisible. Unlike a disease like malaria, which causes characteristic cyclical fevers that indicate their cause, the effects of lead poisoning are more difficult to trace back.
Creating Advanced Market Commitments and Prizes for Pandemic Preparedness
As part of its American Pandemic Preparedness plan, the Biden Administration should establish an interagency working group (IWG) focused exclusively on the design, funding, and implementation of advance market commitments (AMCs) and prizes for vaccine development. Under an AMC, pharmaceutical companies commit to providing many vaccine doses at a fixed price in return for a per-dose federal subsidy. Prizes can support AMCs by rewarding companies for meeting intermediate technical goals.
The IWG would immediately convene experts to identify suitable targets for ambitious vaccine-development and deployment efforts. The group would then work with stakeholders to implement AMCs and prizes crafted around these targets, offering a concrete and durable demonstration of the Administration’s commitment to proactive pandemic preparedness. As the American Pandemic Preparedness plan argues, an important part of rapid vaccine deployment is maintaining “hot manufacturing capacity”. Clear federal AMCs would create the market incentive needed to sustain such capacity, while simultaneously advancing procurement expertise within the federal government, in line with recent recommendations from a government review on the US supply chain.
Challenge and Opportunity
Vaccines are very cost-effective medical interventions that have played a large role in reducing pathogen-induced deaths over the last 200 years. But vaccines do not yet exist for many diseases, including diseases concentrated in the developing world. Vaccines are undersupplied relative to their social benefit because their target populations are often poor and because strong political pressure for lower prices leads to low expected profits. When new vaccines are approved, scaling up production to fully supply low and middle-income countries (LMICs) can take up to 15 years. AMCs solve these issues by incentivizing vaccine development and hastening production scale-up. Prizes play an intermediate role by offering rewards for meeting technical goals along the way.
Vaccine AMCs have a track record of success. In 2007, GAVI, a public-private global health partnership based out of Geneva, launched an AMC for a pneumococcal conjugate vaccine (PCV) that covered pneumococcal strains more common in the developing world. The partnership received its first supply offers in 2009 (a fairly rapid response enabled by the fact that some PCV candidates were already in late-stage clinical trials). Compared to the rotavirus vaccine — which was developed around the same time but did not receive an AMC — PCVs achieved 3–4x greater coverage (defined as the fully vaccinated fraction of the target population). Moreover, new vaccines typically take about 10–15 years to become widely available in LMICs. PCV became available in those countries within a year. This example demonstrates the capacity of AMCs to incentivize rapid scaling. More recently, the United States (through Operation Warp Speed) and several other countries and organizations purchased substantial COVID-19 vaccine doses far in advance of approval, albeit using a more flexible AMC model that prioritized scaling production before data from clinical trials were available.
Plan of Action
To build on the progress and demonstrated success outlined above, the Biden Administration should invest in AMCs and prizes for vaccine development and deployment as part of its American Pandemic Preparedness plan. Below, we detail three specific recommendations for moving forward.
Recommendation 1. Form an Interagency Working Group (IWG) on Rapid Vaccine Innovation
Roles and responsibilities
Vaccine development and manufacturing is a multi-stage process that is too complicated for any single federal agency to manage. The Biden Administration should issue an Executive Order establishing an IWG on Rapid Vaccine Innovation.
Under emergency circumstances, the IWG would be the government hub for time-sensitive vaccine-procurement efforts. Under normal (non-pandemic) circumstances the IWG would focus on extant communicable diseases with a high disease burden and on potential future threats. This latter function would be carried out as follows.
1. Vaccine targeting. A “horizon scanning” IWG subgroup would identify priority targets for rapid vaccine development and broad deployment. The subgroup would consider factors such as pandemic potential, current disease burden, and vaccine tractability. The IWG would also consult with scientists at the VRC (whose work was essential to the rapid development of COVID-19 vaccines, and who already focus on viruses with pandemic potential) and at the CDC (which already performs pathogen surveillance) in making its determinations. Options for initial vaccine targets could include:
- A universal coronavirus vaccine in response to the emergence of potentially immune-evading variants of COVID-19.
- A universal influenza vaccine, like the one already under early-stage development at the National Institutes of Health (NIH).
- A vaccine against Group A streptococcus (GAS). GAS kills about 500,000 people globally annually, mostly through heart and kidney complications or severe infections. Much of this burden falls on LIMCs. GAS also drives high use of antibiotics, which may contribute to antibiotic resistance. A successful AMC for a GAS vaccine would save hundreds of thousands of lives. Fortunately, there are multiplepromising GAS vaccine candidates in early trials. A human-challenge model with potential to accelerate development already exists, and relevant experts and the World Health Assembly acknowledge that GAS prevention should be prioritized. Since two of the leading vaccine candidates are being developed by close U.S. allies (Australia and Canada), prioritizing GAS vaccine development would have the added benefit of strengthening us and our allies as global tensions rise.
- A better tuberculosis vaccine. The technological distance to a better tuberculosis vaccine is greater than the technological distance to a GAS vaccine. But since tuberculosis likely kills twice as many people each year, development of a tuberculosis vaccine would also have a greater payoff.
- An AMC could be deployed to incentivize rapid scale-up of the recently tested malaria vaccine. This could be a flagship program of the United States’ response to China: the Build Back Better World (B3W) initiative, which includes “health and health security” as one of its four priorities. Scaling up deployment of the malaria vaccine in Africa and Southeast Asia would be an excellent way for the United States to regain influence lost in those regions to China’s Belt and Road initiative.
- Recent studies indicate a strong connection between multiple sclerosis and the epstein-barr virus (EBV) and Moderna has recently performed early-stage trials targeting EBV with an mRNA vaccine candidate. Acutely, EBV causes mononucleosis and has been linked with multiple cancers and autoimmune diseases.
- The Strategic National Stockpile (SNS) purchases and stores substantial quantities of vaccines and therapeutics for availability during an emergency. As more countermeasures are developed and then stocked, the financial burden of maintaining the stockpile increases, since expired medications must be replenished over time. There is already an FDA initiative to extend the shelfspan of therapeutics but a targeted strategy to develop vaccines that are shelf-stable for longer and in more varied conditions could reduce the budgetary burden of stockpile maintenance.
2. Incentive design. Once one or more vaccine targets are identified, an IWG subgroup comprising health economists and budget officers would design the AMC(s) and intermediate prizes intended to spur development and deployment of the target(s). Incentive design would (i) be carried out with substantial input from BARDA, which is familiar with the vaccine-manufacturing landscape, and (ii) consider both the technological distance of the target and market competitiveness. An output from this step would be a Vaccine Incentive Roadmap describing the different prizes and incentives that federal agencies will offer to ensure fast, consistent progress towards development and deployment of the target(s) in question. In other words, the linked prizes included in the roadmap will produce sustained incentives for continued forward progress on vaccine development. More information on this roadmap is provided below.
Structure and participation
The IWG should be structured as an integration, with each participating agency providing specific expertise on each aspect of the IWG’s charge. Participants should include senior leaders from the Biomedical Advanced Research and Development Authority (BARDA), the Centers for Disease Control and Prevention (CDC), the Department of Defense (DOD), the Food and Drug Administration (FDA), the U.S. Agency for International Development (USAID), US International Development Finance Corporation (DFC), and the Vaccine Research Center (VRC). BARDA has a track record of successful procurement of vaccines and expertise in negotiating with manufacturers. VRC’s founding mission is vaccine development and it has collaborated with manufacturers on large-scale production for multiple vaccines. They would provide expertise on vaccine tractability. Through upfront guidance on minimum efficacy requirements, the FDA will ensure vaccine standards. FDA will also work with global regulators on the possibility of regulatory reciprocity, akin to their PEPFAR program, which assists low-resource regulators in low and middle-income countries with decision-making.
The IWG should be chaired by a biosecurity expert housed at the White House Office of Science and Technology Policy (OSTP).
Congressional notification
The IWGs recommendations (regarding both targets and AMC/prize design), once finalized, would be submitted to the Senate Health and House Ways and Means Subcommittee to request funding. Because federal agencies must notify Congress if they plan to disburse large prize sums (with agency-specific thresholds), this submittal would also serve as the required formal notification to Congress of prize amounts.
Recommendation 2. Carry out the IWG’s Vaccine Incentive Roadmap
After the IWG has issued its recommendations on vaccine target(s) and incentive (AMC and prizes) design, implementation must follow. Where implementation support comes from will depend on the “technological distance” of the target(s) in question.
Early-stage development focused on in-vitro or animal research should be supported with prizes from BARDA, the Department of Health and Human Services (HHS), and NIH. All federal agencies already have the authority to award prizes under the America Competes Act. Initial prizes could be awarded to vaccine candidates that successfully protect an animal model against disease. Later prizes could be awarded to candidates that hit clinical milestones such as completion of a successful Phase 1 trial in humans. We note that while agencies can theoretically pool funds for a multi-stage prize, cumbersome interagency processes mean that it will likely be easier to have separate agencies fund and oversee the separate prizes included in the roadmap.
Later-stage development should be supported with larger prizes or purchases from USAID and DOD. Once a vaccine candidate has reached early-stage human clinical testing, larger prizes and/or different funding mechanisms will likely be required to advance that candidate to later-stage human testing. This is because costs of moving a vaccine candidate from the preclinical stage to the end of phase 2A (early-stage human clinical testing) range from $14 to $159 million dollars.
It is unlikely that a single federal agency would have the discretionary funds or willingness to sponsor a prize sufficient to incentivize participation in this process. Federal partnerships with private-sector entities and/or philanthropies could supplement federal prize funding. The promise of being a government-approved vendor of a vaccine or a DOD-supported prototype would serve as incentive for external entities to enter into such partnerships. USAID could also leverage its relationships with global health stakeholders and funders to provide incentive funding. Of course, external funding partnerships would be unnecessary if Congress appropriated sufficient designated funding for large vaccine-incentive prizes to relevant agencies.
An alternative to prize funding that would be appropriate for incentivizing later-stage R&D is use of the DOD’s Defense Commercial Solutions Opening (CSO) purchasing authority. DOD could use its CSO authority to pre-purchase vaccine doses in large quantities, effectively creating an AMC. Purchases of up to $100 million can be made through CSO authority. Early prize negotiations would use the leverage provided by becoming a government-approved vendor of vaccines (part of the CSO process) to negotiate for fair prices. A second DOD purchase authority that could be used as an AMC-like incentive is the Other Transaction Authority (OTA), which exempts the DOD from some federal procurement regulations. OTA authority could likely be used to support vaccine research, purchase vaccine prototypes, and pay for some manufacturing of a successful prototype. OTA has also been used to fund research consortia, a possible alternative to a multi-stage prize roadmap. Purchases of up to $20 million can be made through OTA authority. In the context of diseases that affect low and middle income countries, a loan from the US International Development Finance Corporation (DFC) may be an option for supplementing an AMC.
Recommendation 3. Permanently expand BARDA’s mandate to include all communicable diseases, expand BARDA’s funding, and make BARDA the IWG’s permanent home
An IWG is a powerful tool for bringing federal agencies together. With existing prize authority and an administration that prioritizes vaccine development and deployment, much could be accomplished through only the steps outlined above. However, achieving truly transformative results requires a permanent and sustainably funded federal agency to be working consistently on advancing vaccines. Otherwise, future administrations may cancel ongoing IWG projects and/or fail to follow through. As the part of the federal government with the most expertise in therapeutics procurement, BARDA is an ideal permanent home for the IWG’s functions.
BARDA’s mandate is currently limited to biological, chemical, or radiological threats to the health of Americans. This mandate should be expanded to include all important communicable diseases. The newly empowered BARDA would manage public-private partnerships for vaccine procurement, while the NIH would remain the fundamental health-research arm of the U.S. government. Expanding BARDA’s mandate would require Congressional action. Congress would need to amend the Pandemic and All-Hazards Preparedness and Advancing Innovation Act appropriately, and would also need to appropriate specific funding for BARDA to carry out the roles and responsibilities of the IWG over the long term.
Prizes and AMCs only pay out when a product that meets pre-specified requirements is approved, so taxpayers won’t pay for any failures.
For technologically “close” vaccine targets with a high chance of imminent Phase 3 trial success, an AMC incentivizes rapid scale-up of manufacturing and ensures that more doses reach more people sooner. The AMC does this by circumventing a type of “hold-up” problem wherein purchasers negotiate vaccine prices down to per-unit costs. The 2007 GAVI Pneumococcus AMC was of this type. A GAS or malaria vaccine would similarly be “close” targets.
For more technologically distant targets, AMCs should incorporate “kill switches” that give future customers of the vaccine an effective veto over the AMC by way of not paying co-payments. This feature is designed to be a final check on the utility of a vaccine and avoids the difficulty of specifying standards for a vaccine many years ahead of time. An AMC structured in this way works well if a company manufactures a vaccine that meets pre-specified technical details but for hard-to-predict reasons is not useful.
For an especially distant target, a series of prize competitions could substitute for a traditional AMC. In this scenario, an initial prize could be awarded for any vaccine candidates that successfully protect an animal model against disease. A later prize could be awarded to candidates that hit clinical milestones such as completion of a Phase 1 trial in humans.
Other details of AMC and/or prize implementation depend on the market structure and cannot be determined ahead of time. For instance, the optimal AMC design is very different in monopoly versus competitive markets.
Operation Warp Speed spent about $12 billion dollars on COVID-19 vaccine development and purchased hundreds of millions of vaccine doses far in advance of approval or clinical trials. While this was very effective, it is unlikely that Congress would be willing to appropriate such a large sum of money — or see that money disbursed so freely — in non-pandemic situations. A multi-stage prize process still incentivizes vaccine development and deployment but does so for a lower cost.
The government could fund research into market segmentation for vaccines, since many who are vaccine-hesitant are avid consumers of alternative health products/supplements. There may be marketing and promotional strategies inspired by “natural” supplements that can increase vaccine uptake.
The federal government does fund influenza vaccine preparation, but that funding is only for a seasonal flu vaccine that works with 40–60% efficacy: a rate that is well below what other vaccines, such as the measles (97%) and mumps vaccines (88%) achieve. A pandemic influenza with an unexpected genetic background could still catch us by surprise. Investing in a universal influenza vaccine is essential in preparing for that eventuality.
One issue is staffing. Drafting a high-quality AMC contract may require legal and economic expertise that isn’t available in-house at federal agencies, so the administration may need to engage external AMC experts. Another issue may be ensuring that activities outlined herein do not fall between interagency “cracks”. Assigning dedicated staff to oversee each activity will be important. A third issue is the potential for interagency friction. The more agencies that are involved with prize design, the longer it may take to design and authorize a given prize. One possible solution is to have only one agency administer each prize, with informal input from staff in other agencies when required.
Curing Alzheimer’s by Investing in Aging Research
Summary
Congress allocates billions of dollars annually to Alzheimer’s research in hopes of finding an effective prophylactic, treatment, or cure. But these massive investments have little likelihood of paying off absent a game-changing improvement in our present knowledge of biology. Funds currently earmarked for Alzheimer’s research would be more productive if they were instead invested into deepening understanding of aging biology at the cell, tissue, and organ levels. Fundamental research advances in aging biology would directly support better outcomes for patients with Alzheimer’s as well as a plethora of other chronic diseases associated with aging — diseases that are the leading cause of mortality and disability, responsible for 71% of annual deaths worldwide and 79% of years lived with disability. Congress should allow the National Institute on Aging to spend funds currently restricted for research into Alzheimer’s specifically on research into aging biology more broadly. The result would be a society better prepared for the imminent health challenges of an aging population.
Challenge and Opportunity
The NIH estimates that 6.25 million Americans now have Alzheimer’s disease, and that due to an aging population, that number will more than double to 13.85 million by the year 2060. The Economist similarly estimates that an estimated 50 million people worldwide suffer dementia, and that that number will increase to 150 million by the year 2050. These dire statistics, along with astute political maneuvering by Alzheimer’s advocates, have led Congress to earmark billions of dollars of federal health-research funds for Alzheimer’s disease.
President Obama’s FY2014 and FY2015 budget requests explicitly cited the need for additional Alzheimer’s research at the National Institutes of Health (NIH). In FY2014, Congress responded by giving the NIH’s National Institute on Aging (NIA) a small but disproportionate increase in funding relative to other national institutes, “in recognition of the Alzheimer’s disease research initiative throughout NIH.” Congress’s explanatory statement for its FY2015 appropriations laid out good reasons not to earmark a specific portion of NIH funds for Alzheimer’s research, stating:
“In keeping with longstanding practice, the agreement does not recommend a specific amount of NIH funding for this purpose or for any other individual disease. Doing so would establish a dangerous precedent that could politicize the NIH peer review system. Nevertheless, in recognition that Alzheimer’s disease poses a serious threat to the Nation’s long-term health and economic stability, the agreement expects that a significant portion of the recommended increase for NIA should be directed to research on Alzheimer’s. The exact amount should be determined by scientific opportunity of additional research on this disease and the quality of grant applications that are submitted for Alzheimer’s relative to those submitted for other diseases.”
But this position changed suddenly in FY2016, when Congress earmarked $936 million for Alzheimer’s research. The amount earmarked by Congress for Alzheimer’s research has risen almost linearly every year since then, reaching $3.1 billion in FY2021 (Figure 1).
This tsunami of funding has been unprecedented for the NIA. The seemingly limitless availability of money for Alzheimer’s research has created a perverse incentive for the NIH and NIA to solicit additional Alzheimer’s funding, even as agencies struggle to deploy existing funding efficiently. The NIH Director’s latest report to Congress on Alzheimer’s funding suggests that with an additional $226 million per year in funding, the NIH and NIA could effectively treat or prevent Alzheimer’s disease and related dementias by 2025.
This is a laughable untruth. No cure for Alzheimer’s is in the offing. Progress on Alzheimer’s research is stalling and commercial interest is declining. Of the 413 Alzheimer’s clinical trials performed in the United States between 2002 and 2012, 99.6% failed. Recent federal investments seemed to be paying off when in 2021 the Food and Drug Administration (FDA) approved Aduhelm, the first new treatment for Alzheimer’s since 2003. But the approval was based on the surrogate endpoint of amyloid plaques in the brain as observed by PET scans, not on patient outcomes. In its first months on the market, Aduhelm visibly flopped. Scientists subsequently called on the FDA to withdraw marketing approval for the drug. If an effective treatment were likely by 2025, Big Pharma would be doubling down. But Pfizer announced it was abandoning Alzheimer’s research in 2018.
The upshot is clear: lavish funding on treatments and cures for a disease can only do so much absent knowledge of that disease’s underlying biological mechanisms. We as a society must resist the temptation to waste money on expensive shots in the dark, and instead invest strategically into understanding the basic biochemical and genetic mechanisms underlying aging processes at the cell, tissue, and organ levels.
Plan of Action
Aging is the number-one risk factor for Alzheimer’s disease, as it is for many other diseases. All projections of an increasing burden of Alzheimer’s are based on the fact that our society is getting older. And indeed, even if a miraculous cure for Alzheimer’s were to emerge, we would still have to contend with an impending onslaught of other impending medical and social costs.
Economists and scientists have estimated extending average life expectancy in the United States by one year is worth $38 trillion. But funding for basic research on aging remains tight. Outside of the NIA, several foundations in the United States are actively funding aging research: the American Federation for Aging Research (AFAR), The Glenn Foundation for Medical Research, and the SENS Foundation each contribute a few million per year for aging research. Privately funded fast grants have backed bold aging projects with an additional $26 million.
This relatively small investment in basic research has generated billions in private funding to commercialize findings. Startups raised $850 million in 2018 to target aging and age-related diseases. Google’s private research arm Calico is armed with billions and a pharmaceutical partner in Abbvie, and the Buck Institute’s Unity Biotechnology launched an initial public offering (IPO) in 2018. In 2021, Altos Labs raised hundreds of millions to commercialize cellular reprogramming technology. Such dynamism and progress in aging research contrasts markedly with the stagnation in Alzheimer’s research and indicates that the former is a more promising target for federal research dollars.
Now is the time for the NIA to drive science-first funding for the field of aging. Congress should maintain existing high funding levels at NIA, but this funding should no longer be earmarked solely for Alzheimer’s research. In every annual appropriation since FY2016, the House and Senate appropriations committees have issued a joint explanatory statement that has force of law and includes the Alzheimer’s earmark. These committees should revert to their FY2015 position against politically directing NIH funds towards particular ends. The past six years have shown such political direction to be a failed experiment.
Removing the Alzheimer’s earmark would allow the NIA to use its professional judgment to fund the most promising research into aging based on scientific opportunity and the quality of the grant applications it receives. We expect that this in turn would cause agency-funded research to flourish and stimulate further research and commercialization from industry, as privately funded aging research already has. Promising areas that the NIA could invest in include building tools for understanding molecular mechanisms of aging, establishing and validating aging biomarkers, and funding more early-stage clinical trials for promising drugs. By building a better understanding of aging biology, the NIA could do much to render even Alzheimer’s disease treatable.
In 2009, a private task force calling itself the Alzheimer’s Study Group released a report entitled “A National Alzheimer’s Strategic Plan.” The group, co-chaired by former Speaker of the House Newt Gingrich and former Nebraska Senator Bob Kerrey, called on Congress to immediately increase funding for Alzheimer’s and dementia research at the NIH by $1 billion per year.
In response to the report, Senators Susan Collins and Evan Bayh introduced the National Alzheimer’s Project Act (NAPA), which was signed into law in 2011 by Barack Obama. NAPA requires the Department of Health and Human Services to produce an annual assessment of the nation’s progress in preparing for an escalating burden of Alzheimer’s disease. This annual assessment is called the National Plan to Address Alzheimer’s Disease. The first National Plan, released in 2012, established a goal of effectively preventing or treating Alzheimer’s disease by 2025. In addition, the Alzheimer’s Accountability Act, which passed in the 2015 omnibus, gives the NIH director the right and the obligation to report directly to Congress on the amount of additional funds needed to meet the goals of the national plan, including the self-imposed 2025 goal.
Understanding diseases that progress over a long period of time such as Alzheimer’s requires complex clinical studies. Lessons learned from past research indicate that animal models don’t necessarily translate into humans when it comes to such diseases. Heterogeneity in disease presentation, imprecise clinical measures, relevance of target biomarkers, and difficulty in understanding underlying causes exacerbate the problem for Alzheimer’s specifically.
Alzheimer’s is also a whole-system, multifactorial disease. Dementia is associated with a decreased variety of gut microbiota. Getting cataract surgery seemingly reduces Alzheimer’s risk. Inflammatory responses from the immune system can aggravate neurodegenerative diseases. The blood-brain barrier uptakes less plasma protein with age. The list goes on. Understanding Alzheimer’s hence requires understanding of many other biological systems.
Alzheimer’s is named after Alois Alzheimer, a German scientist credited with publishing the first case of the disease in 1906. In the post-mortem brain sample of his patient, he identified extracellular deposits, now known as amyloid plaques, clumps of amyloid-beta (Aβ) protein. In 1991, David Allsop and John Hardy proposed the amyloid hypothesis after discovering a pathogenic mutation in the APP (Aβ precursor protein) gene on chromosome 21. Such a mutation led to increased Aβ deposits which present as early-onset Alzheimer’s disease in families.
The hypothesis suggested that Alzheimer’s follows the pathological cascade of Aβ aggregation → tau phosphorylation → neurofibrillary tangles → neuronal death. These results indicated that Aβ could be a drug target for Alzheimer’s disease.
In the 1990s, Elan Pharmaceuticals proposed a vaccine against Alzhiemer’s by stopping or slowing the formation of Aβ aggregates. It was a compelling idea. In the following decades, drug development centered around this hypothesis, leading to the current approaches to Alzhiemer’s treatment: Aβinhibition (β- and γ-secretase inhibitors), anti-aggregation (metal chelators), Aβ clearing (protease-activity regulating drugs), and immunotherapy.
In the last decade, the growing arsenal of Aβ therapies fueled the excitement that we were close to an Alzheimer’s treatment. The 2009 report, the 2012 national plan, and Obama’s funding requestsseemed to confirm that this was the case.
However, the strength of the amyloid hypothesis has declined since then. Since the shutdown of the first Alzheimer’s vaccine in 2002, numerous other pharmaceutical companies have tried and failed at creating their own vaccine, despite many promising assets shown to clear Aβ plaques in animal models. Monoclonal antibody treatments (of which aducanamab is an example) have reduced free plasma concentrations of Aβ by 90%, binding to all sorts of Aβ from monomeric and soluble Aβ to fibrillar and oligomeric Aβ. These treatments have suffered high-profile late-stage clinical trial failures in the last five years. Similar failures surround other approaches to Alzheimer’s drug development.
There is no doubt these therapies are successful at reducing Aβ concentration in pre-clinical trials. But combined with the continuous failure of these drugs in late-stage clinical trials, perhaps Aβ does not play as major a role in the mechanistic process as hypothesized.
Maintaining Military Medical Readiness Today Saves Lives Tomorrow
Summary
Advances in military medicine are hard won during war but easily lost during peace. Though mortality rates of U.S. troops on the battlefield have improved significantly since World War II, the battlefield mortality rate at the beginning of a war often exceeds the battlefield mortality rate at the end of the previous war. Researchers attribute this phenomenon to erosion, during interwar years, of military readiness to provide combat healthcare. The Perelman School of Medicine at the University of Pennsylvania estimates that better maintaining military medical readiness could have prevented more than 100,000 combat deaths over the past 80 years.1
Loss of life following survivable injury is not unique to the military. Tens of thousands of U.S. civilians succumb to potentially preventable trauma-related deaths every year.2 Since military medical advances are frequently adopted by the civilian healthcare sector,345the White House,working with key federal agencies, should expand military-civilian partnerships (MCPs) in trauma care to achieve a national goal of eliminating preventable deaths. Such an initiative will save lives on the battlefield and the home front — with the ultimate goal of reaching zero preventable deaths.6
Challenge and Opportunity
An unprecedented percentage of service members wounded on the battlefields of Iraq and Afghanistan over the past 20 years made it home to their loved ones. This success is due to the professionalism of our uniformed healthcare providers and their innovations in responding to combat trauma — innovations that include moving blood products closer to the battlefield to lessen the effects of immediate and severe blood loss, deploying resuscitative surgical-system teams close to troops in enemy contact, splitting operations of forward surgical teams in two to increase coverage, and distributing tourniquets to every deployed service member.7
During times of peace, though, our military medical community loses its readiness to save life and limb on the battlefield. Statistics from the “War on Terror” illustrate the tragic consequences that arise when military medical readiness erodes during interwar years. Between October 2001 and June 2011, 4,016 U.S. combat troops died before they reached a military hospital. Of those, 976 (almost 25%) died from what are assessed to be battlefield-survivable injuries.8 A survey of general surgeons who provided deployed casualty care between 2002 and 2012 found that the majority of respondents felt underprepared to meet the demands of battlefield injuries.9
A key reason for interwar deterioration of military medical readiness is that during times of peace, Department of Defense (DoD) priorities shift from treating combat trauma to ensuring the general wellness of active-duty service members, their families, and other beneficiaries at Military Treatment Facilities (MTF) administered by the Defense Health Agency. While beneficiary care is an essential personnel benefit that should not and must not be diminished, it is also essential to recognize that the MHS does not provide sufficient training opportunities to maintain the proficiency of military medical personnel in treating battlefield trauma.10 An independent study conducted by the Institute for Defense Analysis found alarming misalignment between the top ten diagnoses on the battlefield in Iraq and the top 10 diagnoses in MTFs: while the former encompassed a variety of combat-related traumas, the latter were generally less serious (consistent with what one would expect for a predominantly young and healthy patient population). This divergence suggests that the primary missions of uniformed healthcare providers — (1) treating complex combat-related traumas, and (2) serving the needs of a family health practice — are not mutually supportive.11
Recognizing that the MTFs were not providing the necessary trauma-related training to maintain battlefield medical readiness, Congress has directed DoD to establish partnerships with civilian medical academic institutions and major metropolitan hospitals that host level I trauma centers. These partnerships are intended to ensure that the military’s wartime medical specialists12 are continually exposed to the volume and types of complex trauma necessary to ensure they are trained and prepared to rapidly deploy to an area of armed conflict.
These MCPs currently include the U.S. Army Trauma Training Center at Miami Dade Ryder Trauma Center in Florida, California’s U.S. Navy Trauma Training Center at USC/LA County, and the three U.S. Air Force Centers for Sustainment of Trauma and Readiness Skills located at the University of Maryland, the University of Cincinnati, and St. Louis University.13 While individually admirable, these MCPs constitute a patchwork that does not substitute for a coordinated national approach to curbing loss of military and civilian life from potentially survivable injuries. Because of this concern in Congress, section 757 of the FY 2021 NDAA directs DoD to conduct a systematic review of its MCPs to enhance the readiness of the military medical force to provide combat casualty care. The White House, working with the Departments of Defense (DoD), Health and Human Services (HHS), Homeland Security (DHS), and Veterans Affairs (VA), should build on results of the review and move quickly to expand military-civilian partnerships (MCPs) in the context of a national goal of eliminating preventable deaths.
Plan of Action
In 2016, the National Academies of Science, Engineering, and Medicine published a report14 explaining the need to establish a coordinated military/civilian national trauma-care system and presenting an action plan for achieving this goal. Below, we outline an updated, four-part version of the National Academies action plan. These actions will collectively shore up our nation’s military medical readiness, with benefits for American troops and American civilians alike.
Part 1
The White House should reaffirm its commitment to maintaining the quality of healthcare received by DoD beneficiaries, while also establishing national goals of (1) achieving zero preventable deaths from trauma-related injury and (2) minimizing trauma-related disability. It should be clear that these goals align both with the DoD’s mission of ensuring that uniformed medical personnel are prepared to provide battlefield healthcare and with HHS’ objective of strengthening the civilian healthcareworkforce to meet American needs. The White House should encourage partnerships between military and civilian trauma-care units to help achieve this goal.
Part 2
Within six months, the White House should establish a “Zero Preventable Deaths” task force overseen by the White House Office of Science and Technology Policy and cochaired by the HHS Assistant Secretary for Preparedness and Response and the Joint Chiefs of Staff Surgeon. The task force should be responsible for:
- Building on the MCP efforts of the DoD by convening federal agencies (including DHS, the VA, and the Joint Chiefs of Staff) and other governmental, academic, and private-sector stakeholders to identify intermediate objectives, policies, and actions needed to achieve zero preventable deaths.
- Assigning accountability and responsibility for
- Ensuring development of best practices, data standards, and research across the continuum of trauma care,
- Evolving a data-driven research agenda to support trauma care,
- Overcoming any policy or legislative complications resulting from the establishment of the military/civilian national trauma-care system, and
- Executing a strategic communications plan for the effort.
- Securing appropriate congressional appropriations and private sector funding to support the goals of eliminating preventable deaths and minimizing preventable disabilities.
Part 3
The combatant commanders establish the medical requirements for their battle plans and the secretaries of the military departments are responsible for training and equipping their branch’s healthcare professionals to meet these demands. It’s the role of the Secretary of Defense to hold them accountable. The defense secretary does this by:
- Designating the Joint Chiefs of Staff Surgeon to be his representative to the task force.
- Ensuring that military department’s train and equip their forces to the combat- casualty-care personnel and system-support infrastructure requirements of combatant commanders.
- Confirming that the Undersecretary of Defense for Personnel and Readiness and the Assistant Secretary of Defense for Health Affairs have established medical-readiness policy and oversight consistent with the goals of the task force.
- Institutionalizing the military departments support for the military/civilian national trauma-care system.
Part 4
The Secretary of Health and Human Services should position the Assistant Secretary for Preparedness and Response of HHS to lead civilian efforts of the task force. This role includes:
- Coordinating with governmental (federal, state, and local), academic, and private-sector partners to establish a national approach for improving trauma care preparedness for mass-casualty incidents.
- Integrating military/civilian trauma-care partnerships into the American College of Surgeon’s Verification, Review, and Consultation Program.
- Developing and implementing guidelines for establishing an appropriate number, level, and location of MCPs within a region based on the needs of the population and the training requirements of the military departments.
- Taking the leadership role for trauma-care research.
- Developing measures of effectiveness and measures of performance for themilitary/civilian trauma care system.
We have just ended our country’s longest period of war and our military doctors are at their best. If we do not act now, much of what they have learned will be lost and some number of troops will die needlessly on future battlefields.
Yes. During every armed conflict, the uniformed medical community makes incredible advances in preventing the deaths of our troops on the battlefield. MCPs ensure that civilian healthcare providers benefit from those advances.
No. The only priority more important than providing DoD beneficiaries access to the highest-quality healthcare available while the force is in garrison is saving the lives of our soldiers, sailors, airmen, marines, and guardians while the force is on the battlefield. And as discussed above, improving the readiness of military healthcare providers improves quality of care for all Americans.
Yes. But we should ask ourselves if the quality of some of the care delivered in the MTFs could be better. The medical community recognizes that high caseload volumes increase provider experience, which equals better outcomes for patients. But with a few exceptions (usually associated with newborn care, pregnancy, and maternal health), high volumes of work are not characteristic of the MTFs. For example, the consulting group CNA found that the best outcomes for knee replacements are observed in facilities that do 200 or more procedures a year. Only 13% of all knee replacements conducted in MTFs were conducted at MTFs that did 200 or more a year.
FAS Organ Procurement Organization Innovation Cohort Shares Data to Advance Organ Recovery Research
WASHINGTON, D.C.– Today the Federation of American Scientists (FAS) announced that the Organ Procurement Organization (OPO) Innovation Cohort is opening up ten years of data to engage in research to increase the rates of lifesaving organ donations every year.
Data from the U.S. Department of Health and Human Services (HHS) indicate that improvements in organ recovery practices will lead to at least 7,000 additional lifesaving transplants every year.
Bipartisan Congressional leaders have highlighted the need for accelerated data-driven reforms given COVID-19’s ravaging effects on organs. According to a July 19, 2021 letter led by the Senate Finance Committee and the House Committee on Oversight and Reform:
“The COVID-19 pandemic is exacerbating the need for organs now and creating an urgent health equity issue, as communities of color are disproportionately impacted by the failures of the current organ donation system and the effects of COVID-19.”
Historically, OPO accountability and data-driven improvement has been hindered by opacity coupled with self-interpreted and self-reported performance data. The OPO Innovation Cohort will make public a trove of data regarding OPO performance, operations, finances, and governance with the goal of informing ongoing federal policymaking toward improving OPO performance and addressing health inequities. The first tranche of data released will be shared with MIT’s Healthy ML Lab and Wilson Lab, and will include case-level performance data, including all unstructured case notes, allowing for never-before-possible analysis of the organ donation process. Detailed data to be shared as part of the OPO Innovation Cohort can be found here. A visualization of OPO performance can be found here.
MIT’s Healthy ML Lab, led by Dr. Marzyeh Ghassemi, will review the de-identified case notes of seven OPOs, representing one-sixth of the country, to better understand where and how potential donors are lost, including by race and ethnicity. Dr. Ghassemi’s groundbreaking work will include using natural language processing and sentiment analysis of case notes to better understand the ways variation in care, communication and context might impact organ procurement and utilization. The Wilson Lab, led by Dr. Ashia Wilson, will target estimation of risks and opportunities for organ placement by OPOs to improve utilization and fairness of the organ transplant system. Dr. Wilson’s work specializes in using optimization to improve the efficiency and fairness of machine learning systems, and will bring this expertise to look for opportunities to increase the availability of organs for all demographic groups.
“Working with this data is a first step towards making better decisions about how to save more lives through organ procurement and transplantation. We have an opportunity to use machine learning to understand potential issues and lead improvements in transparency and equity,” said Dr. Marzyeh Ghassemi.
“Patients deserve transparency, and this research is even more important given what we are learning about COVID-19’s effects on organs,” said Jennifer Erickson, Senior Fellow, Federation of American Scientists.
The seven organ procurement organizations who are leading in opening up their data include: Donor Network West, Life Connection of Ohio, LiveOn New York, Louisiana Organ Procurement Agency, Mid-America Transplant, OurLegacy, Southwest Transplant Alliance. They have publicly committed to:
- Transparency: public sharing of data/analysis in order to set a standard to which all OPOs can be held;
- Accountability: support for the OPO final rule, and any efforts to move up implementation date so all parts of the country can be served by high-performing OPOs as soon as possible in 2024; and
- Equity: commitment to analyzing/publishing data to ensure all parts of community served.
###
Federal Approval of Over-the-Counter Birth-Control Pills
Summary
Women have a right to contraception, regardless of circumstance. But this right has recently come under threat. Starting in 2016, multiple federal and state regulations pulled critical funding to reproductive and family-planning services. The COVID-19 crisis amplified the challenges Americans face while attempting to receive basic healthcare resources like birth-control pills. To reverse this worrying trend and ensure universal access to contraception in the United States, the federal government should approve over-the-counter (OTC) birth-control pills — thereby removing the need for a prescription to protect women’s health and prevent unintended pregnancies.
Specifically, the Biden-Harris Administration should commission the Food and Drug Administration (FDA) to create an OTC Monograph for oral contraceptives (i.e., birth- control pills). An OTC Monograph is a rulebook established by the FDA that gives specific instructions on the manufacture, distribution and marketing of non- prescription, OTC drugs. Circumstances are right for this action. 2020’s CoronavirusAid, Relief, and Economic Security (CARES) Act established the OTC Monograph Reforms, creating a new and efficient process to produce OTC drugs. The CARES Act also provided the FDA’s Department of Non-prescription Drugs with $110 million over five years1 to produce more OTC drugs. Oral contraceptives are ideal OTC candidates, having been proven safe and effective for 60 years. It is time for the United States to follow the example set by more than 100 countries to date and provide women with OTC birth-control pills.
Empowering Healthy Eating in America
Poor diets present elevated health risks, and Americans need help finding the time and resources to eat nutritiously
Americans get bombarded with promotions for unsubstantiated diet fads on the internet, are exposed to dubious weight-loss branded foods in grocery stores, and often struggle to eat nutritiously. The Dietary Guidelines for Americans recommend a balanced diet of two and a half cups of vegetables, two cups of fruit, six ounces of grains, three cups of dairy, five and a half ounces of protein, and 27 grams of oil every day. This diet is well-balanced, but it is neither practiced by, nor accessible to, all Americans (Figure 1).
Increasing numbers of Americans do not eat healthful diets. In 2018, the National Health and Nutrition Examination Survey found that one in three Americans eats fast food on any given day. Moreover, both rural and urban Americans report that lack of time and access to nutritious foods prevents them from cooking healthy meals. Indeed, a 2017 study indicated that the higher prices of healthy foods – nearly double those of unhealthy foods – can play a role in the U.S. population’s failure to achieve a nutritious diet. When healthy food cost even 14 percent higher than unhealthy food, there was a 24 percent decrease in consuming a high-quality diet. Unfortunately, an unhealthy diet can lead to a variety of health issues, such as obesity, type-2 diabetes, heart disease, and an increased risk of some cancers. To reverse poor health metrics such as the 42.4% of American adults over 20 years of age who suffered from obesity in 2018, policymakers and health experts alike hope to make healthy diets more accessible to all Americans.
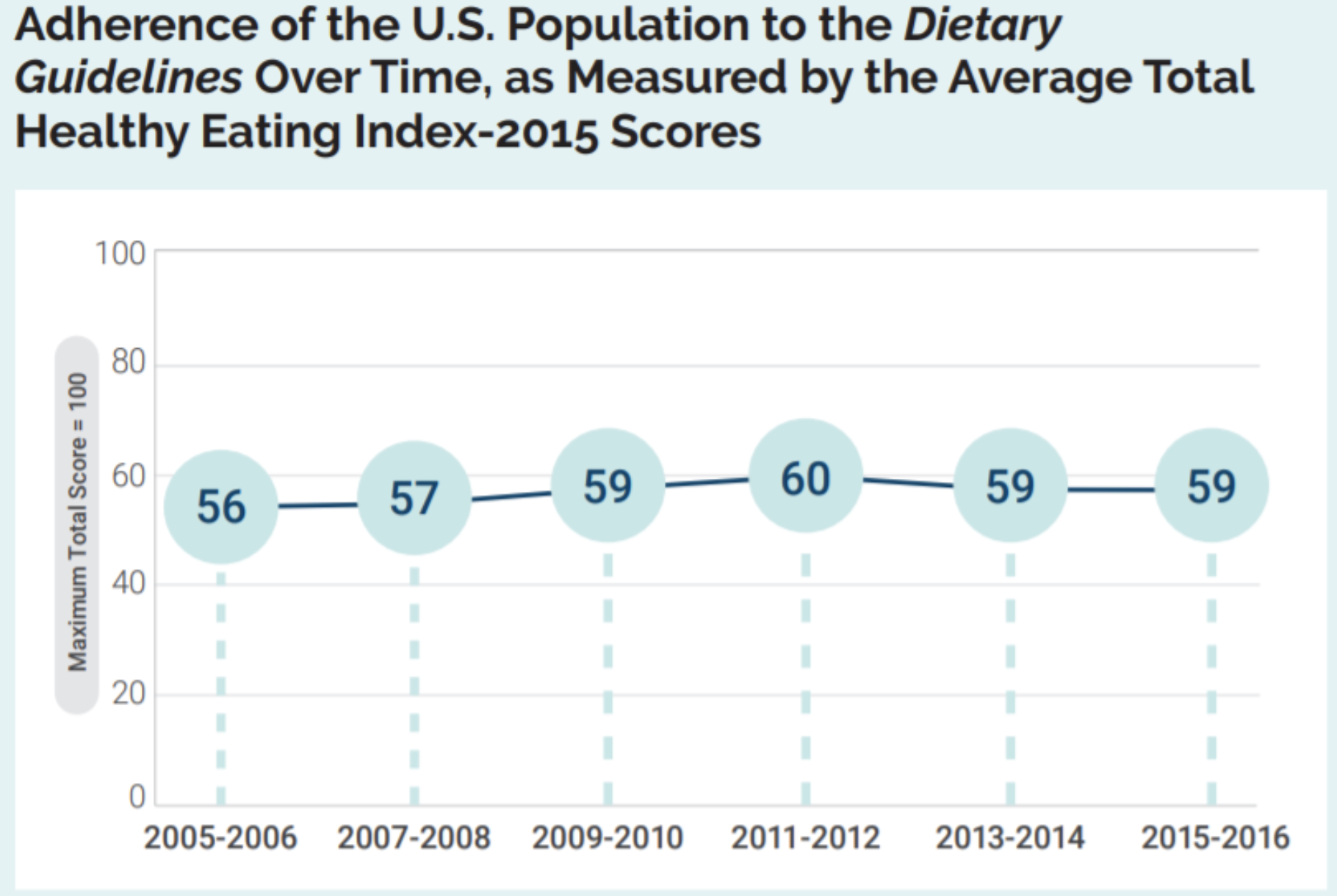
On average, people in the U.S. score between 56 and 60 (out of 100) when evaluated for healthy eating. The maximum test score of 100 points indicates adherence to the American Dietary Guidelines. Figure reproduced from Dietary Guidelines for Americans, 2020-2025.
To empower people to develop more nutritious eating habits, some experts recommend:
- Teaching better practices for caloric intake, which can increase life expectancy;
- Incentivizing healthy eating with financial rewards, such as coupons, when purchasing fruits and vegetables;
- Teaching and encouraging adults to buy and prepare their own meals; and
- Enabling mutual aid initiatives such as community fridges, food banks, and free breakfast programs for those who are food insecure.
For some, the transition to eating a well-balanced diet will require learning how to cook, carving out time to prepare meals, or gaining an understanding of the nutritional value of various foods. In the U.S., there is no justifiable reason people should not be supported by their local, state, and federal governments in efforts to eat healthy.
To improve American dietary habits, policymakers can learn about and implement public health initiatives for nutritional education, as well as break down systemic barriers to healthy eating lifestyles.
This CSPI Science and Technology Policy Snapshot expands upon a scientific exchange between Congressman Bill Foster (D, IL-11) and his new FAS-organized Science Council.